Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Prevalence of Methicillin-Resistant Staphylococcus Aureus in Some Ready-to-Eat Meat Products
*Corresponding author: Fahim A Shaltout, Department of Food Control, Faculty of Veterinary Medicine, Benha University, Egypt
Received: August 12, 2019;Published: August 28, 2019
DOI: 10.34297/AJBSR.2019.05.000855
Abstract
Although Staphylococcus aureus (S. aureus) is a bacterium that remains widely studied because of its high pathogenic potential and its ability to develop resistance to antibiotics routinely used in clinical practice; this study investigated the occurrence of methicillin- resistant Staphylococcus aureus (MRSA) in some ready to eat (RTE) meat products collected from some public restaurants and street vendors in Benha city, Qalubiya governorate, Egypt; a total of 120 RTE beef products represented by kofta, burger, shawerma, and luncheon (30 of each) were examined for the prevalence of S. aureus and molecular detection of MRSA strains represented by the presence of mecA gene containing isolates; results revealed that kofta was the most contaminated samples with S. aureus where the mean count was 5.2x10 CFU\g; followed by burger, shawerma and luncheon samples. Molecular detection of MRSA isolates carrying mecA gene revealed that out of eight examined isolates, 2 (25%) of examined isolates were MRSA strain. The presence of S. aureus especially MRSA strains in high prevalence among examined RTE meat products emphasizes the necessity of enforcing application of strict hygienic measures and GMP during preparation, handling, and serving; in addition, the health authorities must exert more control over street vendors and fast food restaurants.
Keywords: Ready to eat; Meat products; MRSA; PCR; Benha city
Introduction
Now a days, ready to eat (RTE) meat products-based sandwiches of shawerma, kofta, etc. are commonly prepared and sold by many restaurants which are widely distributed all over the country (Takeaway). S. aureus is one of the most important microorganisms which can contaminate or re-contaminate cooked foods by workers hands, equipment or utensils [1]. This microorganism is associated with nosocomial and community-acquired staphylococcal infections, primarily related to the emergence of drug-resistant organisms [2]. Methicillin-resistant S. aureus (MRSA) strains were firstly identified in 1961, immediately after the introduction of methicillin in clinical settings [3]. Since then, increased resistance to methicillin among S. aureus isolates has been observed globally [4]. Because S. aureus is highly prevalent in food and food environments, MRSA may follow the same transmission pattern, and although MRSA infections have not been associated with the consumption of contaminated meats, the pathogen has entered the food chain. Methicillin-resistant S. aureus (MRSA) is mainly attributed to the presence of mecA gene, located on one of Staphylococcal cassette chromosomes mec (SCCmec), that encodes penicillin-binding pro tein 2a (PBP2a) with a low affinity for essentially all beta-lactam antimicrobials resulting in difficult treatment of infections [5]. Methicillin-resistant S. aureus (MRSA) is known to be one of the most prevalent nosocomial pathogens throughout the world and can cause a wide range of food poisoning, pneumonia, postoperative wound infections and nosocomial infections [6]. In recent years, methicillin-resistant S. aureus (MRSA) has been identified in domestic animals and animal-derived food products worldwide [7]. Food products surveyed as meat and its products are widely known to be an important reservoir and main source of MRSA in humans [8]. Therefore, the present study was conducted to investigate the incidence of coagulase-positive S. aureus and methicillin-resistant S. aureus (MRSA) strains in different popular ready-to-eat meat sandwiches (kofta, burger, shawerma, and luncheon) in Benha city.
Materials-and-Methods
Collection of Samples
A grand total of 120 samples of RTE meat products represented by “luncheon, burger, shawerma and kofta” (30 of each) were collected from different restaurants and street vendors in Benha city, Qalubiya governorate, Egypt; Samples were transferred to the laboratory under complete aseptic conditions in ice box within one hour and examined for bacteriological and molecular detection of the incidence of S. aureus and MRSA strains contamination.
Bacteriological Examination
Preparation of sample according to APHA (2013): Twenty- Five grams of the examined samples of meat products were aseptically transferred to a sterile stomacher bag and homogenized with 225 ml of 0.1% sterile buffered peptone water for 1-2 min to give an initial dilution of 1/10. One ml from the original dilution was transferred by means of sterile pipette to another sterile tube containing 9 ml of sterile buffered peptone water (1%), and then mixed thoroughly by using vortex for 5-10 seconds to obtain the next dilution, from which further decimal serial dilutions were prepared.
Determination of Staphylococci and S. aureus count according to (ISO 6888-1:1999, A1:2003): 0.1 ml from each of previously prepared serial dilutions was spread over duplicate large Baired Parker agar plate using a sterile bented glass spreader. The inoculated and control plates were inverted and incubated at 37°C for 48 hours. After which they were examined for colony character. The developed colonies (shiny black colonies) were enumerated and total staphylococcal count/g was calculated. The suspected colonies of Staphylococcus aureus appear as black, shiny, circular, smooth, and convex with narrow white margin and surrounded by a clear zone extending into opaque medium were enumerated and total Staphylococcus aureus count/g was calculated.
Identification of Staphylococcus aureus: Morphological examination [9]. Biochemical identification [10]. Thermostable nuclease test “D-Nase activity” [11]. In-Vitro anti-microbial sensitivity test for isolated S. aureus was performed for investigation of the range of isolates antimicrobial resistance, according to [12].
Molecular detection of MRSA: two isolates of each confirmed coagulase positive S. aureus strains from each examined product were sent to the Central Laboratory for Food Analysis, Faculty of Veterinary Medicine, Benha University, Egypt; and molecularly examined for presence of S. aureus carrying mecA gene (MRSA) using PCR.
Primer sequences of S. aureus used for PCR system following [13] as tabulated Table 1. DNA Extraction using QIA amp kit [14]. Amplification of S. aureus enterotoxin genes [13]. Statistical Analysis results were statistically evaluated by application of Analysis of Variance (ANOVA) test according to [15].
Results
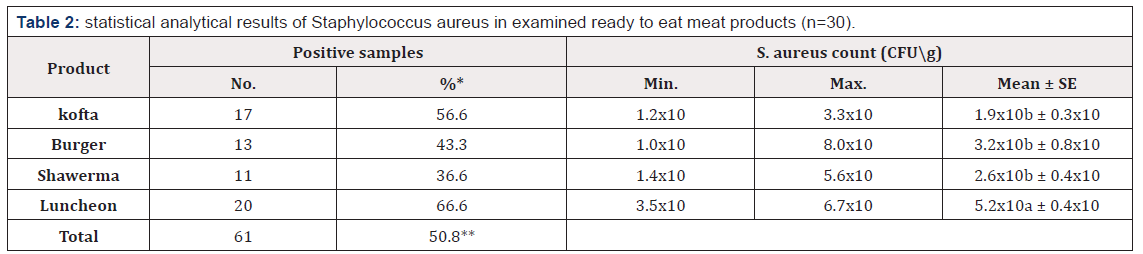
Table 2:statistical analytical results of Staphylococcus aureus in examined ready to eat meat products (n=30).
(ab) values within a column with different superscript letters were significantly different at (P ≤ 0.05). *Percentage in relation to total number of each sample (30). **Percentage in relation to total number of samples (120).
Referring to the results demonstrated Table 2, S. aureus could be isolated from 61 (50.8%) samples, represented by 66.6, 43.3, 36.6, 56.6% with mean counts of 5.2x10, 3.2x10, 2.6x10, and 1.9x10 CFU\g from kofta, burger, shawerma, and luncheon samples, respectively; statistical analysis of variance indicated a significant difference between kofta and the other samples when p ≤ 0.05. In vitro antibiotic sensitivity test was conducted on 61 S. aureus isolates as demonstrated Table 3; although, isolates showed variable sensitivities against different antibiotics, in general, they showed multidrug resistance for about 42.8% of tested antibiotics represented by Oxacillin (70.5%), Methicillin (70.5%), Nalidixic acid (60.6%), Amoxicillin (59.0%), Cefotaxime (55.7%), and Ampicillin (50.8%); while were most sensitive to Norfloxacin (95.1%). Performing of PCR detection of MRSA strains revealed positive detection of mecA gene band at 533bp in two isolates out of examined eight isolates (25%) as presented in Figure 1.
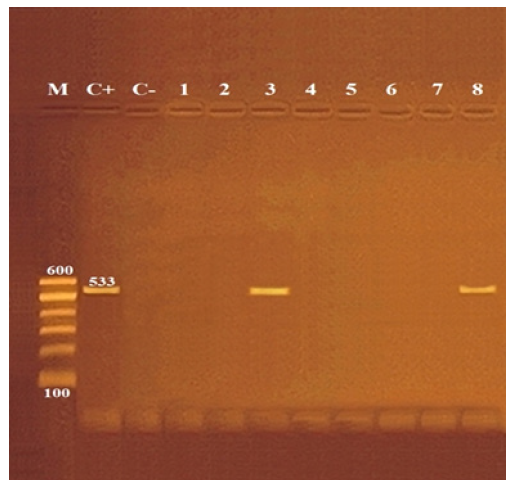
Figure 1:Agarose gel electrophoresis of PCR amplification products of mecA gene for characterization of Methicillin Resistant Staphylococcus aureus (MRSA).
Lane M: 100 bp ladder as molecular size DNA marker.
Lane C+: Control positive S. aureus for mecA gene.
Lane C-: Control negative.
Lanes 3 and 8: Positive S. aureus strains for mecA gene.
Lanes 1, 2, 4, 5, 6 & 7: Negative S. aureus strains for mecA gene.
No.: Number of isolates
%: Percentage in relation to total number of isolates (61).
AA: Antibiogram activity
R: Resistant
S: Sensitive
IS: Intermediate
Discussion
Since few decades ago, S. aureus was reported as incriminated pathogen in 25% of all foodborne illnesses in the United States of America; with continuously misuse of antibiotics and emerging multi-drug resistant bacteria, MRSA strains have been aroused as one of the most feared nosocomial germs that play important role in food poisoning; and however low prevalence of MRSA in food, the thread comes from difficulties of treating of infections due to multidrug resistance of MRSA [16-18].
Recent results revealed that, luncheon samples (consumed immediately without pre heat-treatment) recorded lower S. aureus counts than examined pre-consumption heated treated samples (kofta, burger, and shawerma); it may be referred to under cooking or improper heat treatment, handling, and\or added chemical preservatives to luncheon during processing that play a direct powerful antimicrobial action against S. aureus.
Tabulated results of Table 1 were somewhat agreed with those reported [19,20], who recorded that the incidence of S. aureus in their examined RTE kofta, burger, shawerma, and luncheon was 60, 46.6, 40, and 60%, respectively. The obtained results have been lower than those recorded by [6,19,21] who recorded that the mean S. aureus counts in examined shawerma, kofta, luncheon, and burger samples was 3.9 ×103, 6.4 ×103, 2.5x103, and 1.9x103 CFU\g, respectively; while, they were higher than those recorded by [22-24] who detected S. aureus in 35, 25, 25, 8.6% of examined kofta, burger, shawerma, and luncheon samples, respectively.
Variations between authors may be attributed to the differences in manufacturing, processing and handling procedures. Presence of S. aureus in such RTE foods highlighted preparation, handling, storage or service faults which may come through cross-contamination from raw food, food handlers and the surrounding environment; in addition, spices, equipment, dressings, knives, and other additives are considered as the source of contamination.
Results of antimicrobial sensitivity test as summarized in Table 2 were somewhat agreed with the results recorded by [25,26] who recorded a multidrug resistance of their S. aureus isolated from meat and meat products. Most of S. aureus isolates were resistant to all β-lactams antibiotics, which is conferred by the mecA gene, which codes for an altered penicillin-binding protein (PBP2a or PBP20) that has a lower affinity for binding β-lactams (penicillins, cephalosporins, and carbapenems). This allows resistance to all β-lactam antibiotics and obviates their clinical use during MRSA infections as mentioned by [27]. From the other hand, results of molecular detection of the presence of MRSA in examined RTE samples agreed with [19,21] who could detect mecA gene containing S. aureus isolates from examined RTE samples [28-30].
Conclusion
The high prevalence of S. aureus among the tested samples, mainly in kofta and luncheon samples, and the presence of the MRSA in prepared foods highlighted the necessity of enforcing hygienic practices within fast food and street vended foods kitchens. In the future, the molecular and ecological characterization of isolated MRSA strains must be performed to determine the origin of contamination. Better knowledge of strict hygienic practices during collection of raw materials, preparation of food, holding, storage and serving must be educated to food handlers.
Acknowledgement
Authors pleased to thank all members of food control department, Faculty of Veterinary Medicine, Benha University and members of Animal Health Research Institute, Benha branch for their kindly support and encouragement
References
- Bryan FL (1988) Risks associated with vehicles of foodborne pathogens and toxins. J Food Protection 51(6): 498-508
- DeLeo FR, Chambers HF (2009) Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J Clinical Investigations 119(9): 2464-2474
- Barber M (1961) Methicillin-resistant staphylococci. J Clinical Pathology 14(4): 385-393.
- Chambers HF (1997) Methicillin-resistance in staphylococci: molecular and biochemical basis and clinical implications. Clinical Microbiology Reviews 10(4): 781-791.
- Thaker HC, Brahmbhatt MN, Nayak JB (2013) Isolation and identification of Staphylococcus aureus from milk and milk products and their drug resistance patterns in Anand, Gujarat. Veterinary World J 6 (1): 10- 13.
- Khosravi AD, Jenabi A, Montazeri EA (2017) Distribution of genes encoding resistance to aminoglycoside modifying enzymes in methicillin-resistant Staphylococcus aureus (MRSA) strains. Kaohsiung J Medical Sciences 33(12): 587-593.
- Hanson BM, Dressler AE, Harper AL, Scheibel RP, Wardyn SE, et al. (2011) Prevalence of S. aureus (MRSA) on retail meat in Iowa. J. Infection and Public Health 4(4): 169-174.
- Contreras CP, Nunes Da Silva LN, Ferreira DC, Ferreira JD, Almeida RC (2015) Prevalence of methicillin-resistant Staphylococcus aureus in raw hamburgers and ready-to-eat sandwiches commercialized in supermarkets and fast food outlets in Brazil. J Food and Nutrition Sciences 6: 1324-1331
- Cruickshank R, Duguid J, Marmion B, Swain RH (1975) Medical Microbiology. 12th Edn, Edinburg, London and New York.
- MacFaddin JF (2000) Biochemical tests for identification medical bacteria. Warery Press Inc, Baltimore, 21202 USA
- Lachia R, Genigeogis C, Hoeprich P (1971) Metachromatic agar-diffusion methods for detecting Staphylococcal nuclease activity. Appled and Environmental Microbiology 21(4): 585-587
- Konemann E, Allen S, Janda W, Schreckenberger C, Winn W (1997) Color Atlas and Textbook of Diagnostic Microbiology. 5th Edn, Lippincott, Philadelphia, New York
- Jukes L, Mikhail J, Naledi B, Hadfield SJ, Harris LG et al. (2010) Rapid differentiation of Staphylococcus aureus, Staphylococcus epidermidis and other coagulase-negative staphylococci and methicillin susceptibility testing directly from growth-positive blood cultures by multiplex real-time PCR. J Medical Microbiology 59(Pt 12): 1456-1461
- Shah D, Shringi S, Besser T, Call D (2009) Molecular detection of foodborne pathogens, Boca Raton, CRC Press In: Liu D(Ed) Taylor & Francis group, Florida, USA. Pp. 369-389.
- Feldman D, Ganon J, Haffman R, Simpson J (2003) The solution for data analysis and presentation graphics. 2nd Edn, Abacus Lancripts, Berkeley, USA.
- Bean NH, Goulding JS, Matthew TD, Angulo FJ (1997) Surveillance for foodborne disease outbreaks-United States, 1988-1992. J Food Protection 60(10): 1265-1286
- Cha JD, Choi SM, Park JH (2014) Combination of acacetin with antibiotics against methicillin resistant Staphylococcus aureus isolated from clinical specimens. Advances in Bioscience and Biotechnology 5(4): 398-408
- Sciezynska H, Mackiw E, Maka L, Pawlowska K (2012) The new microbiological hazards in food. Roczniki Panstwowego Zakladu Higieny 63(4): 397-402
- Laban RA (2018) Detection of methicillin-resistant Staphylococcus aureus in some meat products. Thesis, Master of Veterinary Medicine, Zagazig University, Egypt
- Rawash RA (2015) Bacteriological status of some ready to eat meat and poultry meat meals in Benha city. Thesis, Master of Veterinary Medicine, Benha University, Egypt
- Morshdy AMA, Hussein MA, Tharwat AE, Fakhry BA (2018) Prevalence of enterotoxigenic and multi-drug resistant Staphylococcus aureus in ready to eat meat sandwiches. Slovenian Veterinary Research 55(20): 367-374
- Abd Allah-Enas MA (2011) Microbial and chemical evaluation of fast foods. Thesis, Master of Veterinary Medicine, Benha University, Egypt
- Ali SFH, Abd-El-Aziz DM (2011) Incidence of enterotoxigenic Staphylococcus aureus in some ready-to-eat sandwiches in Assuit city with special reference to methicillin resistant Staphylococcus aureus strains. Assiut Veterinary Medicine Journal 57(129): 95-106.
- Heweidy AY (2016) Prevalence of some foodborne micro-organisms in meat and meat products. Thesis, Master of Veterinary Medicine, Benha University, Egypt.
- Bahbah EA (2019) Prevalence of staphylococci in meat products with special reference methicillin-resistant Staphylococcus aureus (MRSA) in Kaliobia governorate. Thesis, Master of Veterinary Medicine, Benha University, Egypt
- Hosny DMA (2016) Characterization of methicillin-resistant Staphylococcus aureus (MRSA) isolated from food products of poultry origin in Egypt. Thesis, Master of Veterinary Medicine, Benha University, Egypt
- Chambers HF (2001) The changing epidemiology of Staphylococcus aureus? Emergence of Infectious Diseases 7(2): 178-182.
- Abd Allah-Mona I (2016) The proportion of the presence of resistant strains of S. aureus isolated from some meat products to antibiotics. Thesis, Master of Veterinary Medicine, Benha University, Egypt
- American Public Health Association “APHA” (2013) Compendium of methods for the microbiological examination of food. T Matthew Taylor et al (Eds) 4th Edn, 2 Washington DC, USA
- ISO 6888-1:1999, A1:2003. Microbiology of food and animal feeding stuffs-Horizontal method for the enumeration of coagulase-positive staphylococci (Staphylococcus aureus and other species)-Part 1: Technique using Baird-Parker agar medium AMENDMENT 1: Inclusion of precision data.




 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.