Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Calcium Dysregulation and Mitochondrial Dysfunction Form A Vicious Cycle in Parkinson’s Disease
*Corresponding author: Angelika B Harbauer, Max-Planck-Institute for Neurobiology, Institute of Neuronal Cell Biology, Technische Universität München, Munich, Germany
Received:September 18, 2019;Published: September 26, 2019
DOI: 10.34297/AJBSR.2019.05.000920
Abstract
Mitochondria are not only the “power houses of the cell” but also function as a major Ca2+ buffer in the cell. Mitochondrial dynamics respond to the level of Ca2+ in the cell in order to maintain a beneficial feedback cycle between Ca2+ buffering and mitochondrial dynamics that allows adaption of mitochondrial to the cellular (sub) environment. Mutations in proteins linked to Parkinson’s disease (PD) as well as are linked to both mitochondrial dysfunction and Ca2+ dysregulation, which can trap the cell in a vicious cycle. The high energetic demands and high Ca2+ of dopaminergic neurons may explain why this cell type is the most vulnerable to mutations in PD related genes.
Introduction
Neurons depend on a healthy pool of mitochondria, as they require large amounts of energy and Ca2+ buffering, yet their highly extended, complex structures and long axons [1] make maintaining and distributing mitochondria a challenging task [2]. Parkinson’s disease (PD has long been linked to mitochondrial dysfunction [3], yet why specifically dopaminergic neurons degenerate first remains to be elucidated.
Regulation of mitochondrial dynamics
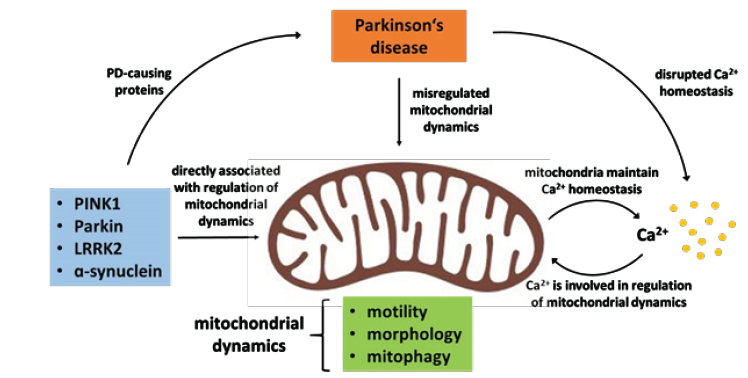
Mitochondrial maintenance and proper axonal distribution in neurons are possible due to the highly dynamic nature of these organelles. Almost all mitochondrial proteins are post-translationally imported into the organelle [4] and mitochondrial trafficking distributes them along the cytoskeleton across the cell [5,6]. Changes in mitochondrial morphology by fusion and fission events further adjust their functionality [7,8] and finally clearance of damaged mitochondria via mitophagy, a specific form of autophagy, contributes to maintaining a pool of healthy mitochondria [9-11]. All these processes are highly regulated to ensure proper mitochondrial func tion in response to the (sub-)cellular environment (Protein import: [4,12]; Trafficking: [9,13-16]; Fission/fusion: [17-19]; Mitophagy: [20-22]. While mitophagy has received a lot of attention as two of the main players are mutated in hereditary PD (PINK1/Parkin) [23,24], several signalling pathways disrupted in PD also alter mitochondrial trafficking (Figure 1).
The blue box lists those proteins found mutated in PD, which directly influence one or more aspects of mitochondrial dynamics and Ca2+ handling. The aspects of mitochondrial dynamics regulated are listed in the green box. The beneficial feedback cycle between Ca2+ and mitochondrial dynamics that allows adaption of mitochondrial dynamics to the cellular (sub)environment is depicted on the right. Upon misregulation of either side of the feedback loop a vicious cycle ensues that exacerbates both mitochondrial dysfunction and disrupted Ca2+ signalling in PD.
A central target of these pathways is the mitochondrial outer membrane protein Miro (also RHOT1/2) which couples the organelle to the cytoskeleton via the adaptor protein Milton (also TRAK1/2) [25-28]. Miro gets phosphorylated by the kinase PINK1 and consecutively ubiquitinated by the E3-Ligase Parkin [20-22], which leads to its degradation and mitochondrial arrest [14,29]. A parallel pathway leading to Miro degradation seems to involve LRRK2 kinase [30]. Likewise, accumulated a-synuclein also dysregulates Miro on several levels. It affects Miro subcellular distribution and limits transport of healthy mitochondria into axons [31]. Furthermore, it also prevents Miro degradation in response to mitochondrial damage [32]. Finally, cytosolic Ca2+ directly binds to the EF hands in Miro, leading to a conformational change that also arrests mitochondrial movement [13,33-35].
Calcium signalling in PD
Ca2+ is essential for cellular signalling, as it allows cells to quickly adapt and respond to the local microenvironment. The failure to control Ca2+ has devastating consequences for the cell, eventually leading to cell death [36]. Neuronal mitochondria are especially needed to buffer cytosolic Ca2+ transients elicited by neuronal activity [37]. In this way, they both prolong the signals and protect neurons from the possibly detrimental Ca2+ spikes. Hence, it is not surprising that disruption of Ca2+ homeostasis is a pathological feature of several neurodegenerative diseases including PD [38]. Several PD-causing proteins are involved in controlling Ca2+ homeostasis such as PINK1, Parkin, LRRK2 and a-synuclein [39-42]. Ca2+ mito uptake, mainly mediated by the mitochondrial calcium uniporter (MCU), is required for maintaining Ca2+ homeostasis [43,44]. Furthermore, the mitochondrial inner membrane Ca2+/H+ antiporter LETM1 is involved in Ca2+ mito uptake. PINK1 phosphorylates LETM1, thereby regulating mitochondrial Ca2+ levels [42]. Loss of PINK1 has been shown to result in disrupted mitochondrial Ca2+ transport and make neurons more vulnerable to stress [42,45].
Miro at the center of mitochondrial calcium regulation
Miro, the mitochondrial outer membrane protein, involved in mitochondrial transport has recently also been reported to be involved in Ca2+ -related processes: Regulation of Ca2+ uptake at ER mitochondria encounter structures (ERMCS) and control of mitochondrial shape in response to Ca2+.
The Ca2+ uptake into mitochondria mainly takes place at ERMCS [46]. Miro has been found to be involved in regulating Ca2+ transfer from ER to mitochondria independent of its function in mitochondrial transport and morphology. Its yeast homolog Gem1p is translocated to ERMCS regulating the number and the size of these complexes [47]. This localization is conserved in mammalian Miro [47]. A more recent study confirms the role of Miro in controlling Ca2+ mito homeostasis at ERMCS [48]. Once Miro is translocated to ERMCS, it interacts with ERMCS components modulating the Ca2+ transfer and the integrity of the complex. The localization of Miro to ERMCS is promoted by Polo kinase-mediated phosphorylation of Miro. Loss of Miro results in Ca2+ mito depletion and overexpression in Ca2+ mito overload [48] revealing the importance of the Polo/Miro signalling pathway in the regulation of Ca2+ mito uptake. Furthermore, this study shows that both PINK1 and LRRK2 are involved in this process. They function upstream of Miro and seem to control the Ca2+ mito homeostasis through Miro. In Drosophila PINK1 and LRRK2 G2019S mutants, Ca2+ mito homeostasis is dysregulated and characterized by elevated Ca2+ mito levels. Upregulated Miro levels have been found to be responsible for the dysregulation in the PD models [48]. This is in line with the fact that both PINK1 and LRRK2 are involved in pathways that result in degradation and thus downregulation of Miro [14,30]. The increased Ca2+ mito levels, present in PINK1 and LRRK2 mutants, lead to mitochondrial swelling and eventually neuronal death.
Finally, mitochondria undergo a morphology change called MiST mediated by Miro sensing Ca2+ [49]. Miro-dependent MiST is involved in mitochondrial quality control as it is required for autophagy and mitophagy. Considering the disrupted Ca2+ homeostasis in PD, MiST may be mistakenly triggered by pathologically increased cytosolic Ca2+ levels, thereby contributing to mitochondrial dysfunction.
Why are dopaminergic neurons so susceptible?
It has been shown that, with age, the amount of Ca2+ dysregulation increases [50]. Ca2+ dysregulation, however, is not ubiquitous but restricted to specific cell types. Dopaminergic neurons, the main cell type affected in PD, are autonomous pacemakers relying on L-type Ca2+ channels. It has been shown that aging is associated with increased reliance of dopaminergic neurons on these Ca2+ channels accompanied by sustained increase in cytosolic Ca2+ levels [51]. With the need to pump Ca2+ out of the cell come higher energy demands and thus an increased oxidative phosphorylation (OXPHOS) rate. The increased need to buffer Ca2+ and therefore elevat ed Ca2+ mito as well as the increased OXPHOS rate result in elevated mitochondrial oxidative stress [52,53]. Ca2+ mito overload may even directly trigger opening of the permeability transition pore (PTP) leading to the release of proapoptotic factors and even more Ca2+ into the cytosol [54]. This further exacerbates the mitochondrial phenotype due to Ca2+ dependent arrest in a vicious cycle (Figure 1) and will eventually lead to programmed cell death [55,56]. This, along with the need to feed an extensively branched axonal arbor [57], may be one of the reasons why dopaminergic neurons are particularly susceptible in PD
References
- Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, et al. (2009) Single Nigrostriatal Dopaminergic Neurons Form Widely Spread and Highly Dense Axonal Arborizations in the Neostriatum. J Neurosci 29(2): 444-453.
- Misgeld T, Schwarz TL (2017) Mitostasis in Neurons: Maintaining Mitochondria in an Extended Cellular Architecture. Neuron 96(3): 651-666.
- Exner N, Lutz AK, Haass C, Winklhofer KF (2012) Mitochondrial dysfunction in Parkinson’s disease: molecular mechanisms and pathophysiological consequences. EMBO J 31(14): 3038-3062.
- Harbauer AB, Zahedi RP, Sickmann A, Pfanner N, Meisinger C (2014) The protein import machinery of mitochondria-a regulatory hub in metabolism, stress, and disease. Cell Metab 19(3): 357-372.
- Saxton WM, Hollenbeck PJ (2012) The axonal transport of mitochondria. Journal of Cell Science 125(Pt 9): 2095-2104.
- Schwarz TL (2013) Mitochondrial trafficking in neurons. Cold Spring Harb Perspect Biol 5(6): 1-15.
- Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, et al. (2009) SLP-2 is required for stress induced mitochondrial hyperfusion. EMBO J 28(11): 1589-1600.
- Rolland SG, Motori E, Memar N, Hench J, Frank S, et al. (2013) Impaired complex IV activity in response to loss of LRPPRC function can be compensated by mitochondrial hyperfusion. Proc Natl Acad Sci U S A 110(32): E2967-2976.
- Ashrafi G, Schwarz TL (2013) The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ 20(1): 31-42.
- Pickrell AM, Huang CH, Kennedy SR, Ordureau A, Sideris DP, et al. (2015) Endogenous Parkin Preserves Dopaminergic Substantia Nigral Neurons following Mitochondrial DNA Mutagenic Stress. Neuron 87(2): 371-381.
- Eiyama A, Okamoto K (2015) PINK1/Parkin-mediated mitophagy in mammalian cells. Curr Opin Cell Biol 33: 95-101.
- Kravic B, Harbauer AB, Romanello V, Simeone L, Vögtle FN, et al. (2018) In mammalian skeletal muscle, phosphorylation of TOMM22 by protein kinase CSNK2/CK2 controls mitophagy. Autophagy 14(2): 311-335.
- Wang X, Schwarz TL (2009) The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell 136(1): 163-174.
- Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, et al. (2011) PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell 147(4): 893-906.
- Pekkurnaz G, Trinidad JC, Wang X, Kong D, Schwarz TL (2014) Glucose Regulates Mitochondrial Motility via Milton Modification by O-GlcNAc Transferase. Cell 158(1): 54-68.
- Yu SB, Pekkurnaz G (2018) Mechanisms Orchestrating Mitochondrial Dynamics for Energy Homeostasis. J Mol Biol 430(21): 3922-3941.
- Cerveny KL, Tamura Y, Zhang Z, Jensen RE, Sesaki H (2007) Regulation of mitochondrial fusion and division. Trends Cell Biol 17(11): 563-569.
- Wang F, Liu P, Zhang Q, Zhu J, Chen T, et al. (2012) Phosphorylation and ubiquitination of dynamin-related proteins (AtDRP3A/3B) synergically regulate mitochondrial proliferation during mitosis. Plant J 72(1): 43-56.
- Cribbs JT, Strack S (2007) Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep 8(10): 939-944.
- Durcan TM, Fon EA (2015) The three ‘P’s of mitophagy: PARKIN, PINK1, and post-translational modifications. Genes Dev 29(10): 989-999.
- Pickrell AM, Youle RJ (2015) The Roles of PINK1, Parkin, and Mitochondrial Fidelity in Parkinson’s Disease. Neuron 85(2): 257-273.
- Yamano K, Matsuda N, Tanaka K (2016) The ubiquitin signal and autophagy: an orchestrated dance leading to mitochondrial degradation. EMBO Rep 17(3): 300-316.
- Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, et al. (2007) Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci U S A 104(27): 11441-11446.
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MMK, Harvey K, et al. (2004) Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 304(5674): 1158-1160.
- Fransson S, Ruusala A, Aspenström P (2006) The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem Biophys Res Commun 344(2): 500-510.
- Glater EE, Megeath LJ, Stowers RS, Schwarz TL (2006) Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol 173(4): 545-557.
- van Spronsen M, Mikhaylova M, Lipka J, Schlager MA, van den Heuvel DJ, et al. (2013) TRAK/Milton motoradaptor proteins steer mitochondrial trafficking to axons and dendrites. Neuron 77(3): 485-502.
- Stowers RS, Megeath LJ, Górska Andrzejak J, Meinertzhagen IA, Schwarz TL (2002) Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron 36(6): 1063-1077.
- Liu S, Sawada T, Lee S, Yu W, Silverio G, et al. (2012) Parkinson’s disease-associated kinase PINK1 regulates Miro protein level and axonal transport of mitochondria. PLoS Genet 8(3): e1002537.
- Hsieh CH, Shaltouki A, Gonzalez AE, Bettencourt da Cruz A, Burbulla LF, et al. (2016) Functional Impairment in Miro Degradation and Mitophagy Is a Shared Feature in Familial and Sporadic Parkinson’s Disease. Cell Stem Cell 19(6): 709-724.
- Prots I, Grosch J, Brazdis RM, Simmnacher K, Veber V, et al. (2018) α-Synuclein oligomers induce early axonal dysfunction in human iPSC-based models of synucleinopathies. Proc Natl Acad Sci U S A 115(30): 7813-7818.
- Shaltouki A, Hsieh CH, Kim MJ, Wang X (2018) Alpha-synuclein delays mitophagy and targeting Miro rescues neuron loss in Parkinson’s models. Acta Neuropathol 136(4): 607-620.
- Chen Y, Sheng ZH (2013) Kinesin-1-syntaphilin coupling mediates activity-dependent regulation of axonal mitochondrial transport. J Cell Biol 202(2): 351-364.
- Macaskill AF, Rinholm JE, Twelvetrees AE, Arancibia Carcamo IL, Muir J, et al. (2009) Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron 61(4): 541-555.
- Saotome M, Safiulina D, Szabadkai G, Das S, Fransson A, et al. (2008) Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc Natl Acad Sci U S A 105(52): 20728-20733.
- Wojda U, Salinska E, Kuznicki J (2009) Calcium ions in neuronal degeneration. IUBMB Life 60(9): 575-590.
- Werth JL, Thayer SA (1994) Mitochondria buffer physiological calcium loads in cultured rat dorsal root ganglion neurons. J Neurosci 14(1): 348-356.
- Zaichick S V, McGrath KM, Caraveo G (2017) The role of Ca 2+ signaling in Parkinson’s disease. Dis Model Mech 10(5): 519-535.
- Calì T, Ottolini D, Negro A, Brini M (2013) Enhanced parkin levels favor ER-mitochondria crosstalk and guarantee Ca(2+) transfer to sustain cell bioenergetics. Biochim Biophys Acta 1832(4): 495-508.
- Calì T, Ottolini D, Negro A, Brini M (2012) α-Synuclein controls mitochondrial calcium homeostasis by enhancing endoplasmic reticulum-mitochondria interactions. J Biol Chem 287(22): 17914-17929.
- Lee KS, Huh S, Lee S, Wu Z, Kim AK, et al. Altered ER–mitochondria contact impacts mitochondria calcium homeostasis and contributes to neurodegeneration in vivo in disease models. Proc Natl Acad Sci U S A 115(38): E8844-E8853.
- Huang E, Qu D, Huang T, Rizzi N, Boonying W, et al. (2017) PINK1-mediated phosphorylation of LETM1 regulates mitochondrial calcium transport and protects neurons against mitochondrial stress. Nat Commun 8(1): 1399.
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher Timme CA, et al. (2011) Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476(7360): 341-345.
- De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R (2011) A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476(7360): 336-340.
- Gandhi S, Wood Kaczmar A, Yao Z, Plun Favreau H, Deas E, et al. (2009) PINK1-Associated Parkinson’s Disease Is Caused by Neuronal Vulnerability to Calcium-Induced Cell Death. Mol Cell 33(5): 627-638.
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, et al. (1998) Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280(5370): 1763-1766.
- Kornmann B, Osman C, Walter P (2011) The conserved GTPase Gem1 regulates endoplasmic reticulum-mitochondria connections. Proc Natl Acad Sci U S A 108(34): 14151-14156.
- Lee S, Lee KS, Huh S, Liu S, Lee DY, et al. (2016) Polo Kinase Phosphorylates Miro to Control ER-Mitochondria Contact Sites and Mitochondrial Ca2+ Homeostasis in Neural Stem Cell Development. Dev Cell 37(2): 174-189.
- Nemani N, Carvalho E, Tomar D, Dong Z, Ketschek A, et al. (2018) MIRO-1 Determines Mitochondrial Shape Transition upon GPCR Activation and Ca2+ Stress. Cell Rep 23(4): 1005-1019.
- Kumar A, Bodhinathan K, Foster TC (2009) Susceptibility to Calcium Dysregulation during Brain Aging. Front Aging Neurosci 1: 2.
- Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, et al. (2007) ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature 447(7148): 1081-1086.
- Pacelli C, Giguère N, Bourque MJ, Lévesque M, Slack RS, et al. (2015) Elevated Mitochondrial Bioenergetics and Axonal Arborization Size Are Key Contributors to the Vulnerability of Dopamine Neurons. Curr Biol 25(18): 2349-2360.
- Surmeier DJ, Guzman JN, Sanchez Padilla J, Goldberg JA (2010) What causes the death of dopaminergic neurons in Parkinson’s disease? Progress in brain research pp. 59-77.
- Rasola A, Bernardi P (2007) The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis 12(5): 815-833.
- Kroemer G, Galluzzi L, Brenner C (2007) Mitochondrial Membrane Permeabilization in Cell Death. Physiol Rev 87(1): 99-163.
- Hunter DR, Haworth RA, Southard JH (1976) Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem 251(16): 5069-5077.
- Bolam JP, Pissadaki EK (2012) Living on the edge with too many mouths to feed: why dopamine neurons die. Mov Disord 27(12): 1478-1483.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.