Mini Review 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Cell Cycle Dynamics and Endoreplication In the Mosquito Midgut
*Corresponding author: Fidel de la Cruz Hernandez Hernandez, Department of Infectomics and Molecular Pathogenesis, Center for Research and Advanced Studies of the National Polytechnic Institute (CINVESTAV), Humberto Lanz-Mendoza, Center for Infectious Diseases Research, Cuernavaca,National Institute of Public Health (INSP), Mexico.
Received: August 27, 2019;Published: September 04, 2019
DOI: 10.34297/AJBSR.2019.05.000871
Abstract
The mosquitoes are the major transmissors of vector-borne infections, including virus, protozoans and helminths and, during the interaction it is critical for pathogens to accomplish immune evasion actions whereas the mosquito turn on immune mechanisms for its survival. The mosquito midgut is the organ where initial interactions with pathogens happen, and the insect has mechanisms to repair the tissue and to eliminate the invaders. For these tasks’ mosquitoes use different strategies including the specific DNA replication, amplifying specific genes and without cell division in order to have more DNA templates for transcription that allow, in a secondary infection, the fast production of immune proteins in a process known as priming. The study of these processes is necessary for the understanding of the mechanisms during insect-pathogens interactions, and to design future interventions to block mosquito pathogens transmission that could be environment-friendly and not depending on costly chemicals.
Introduction
The mosquito midgut is an essential tissue where pathogens and microorganisms interact. This organ is fundamental to transmit diverse diseases, including the virus causing dengue, zika, chikungunya, and the malaria causing parasite Plasmodium sp. During the interaction with pathogens, the midgut cells react; repairing cell damage and producing immune molecules to fight against invaders. However, we have limited knowledge of the mechanisms behind the response and the cell-damaged repair. The cell cycle is a finely regulated process that manages proliferation and differentiation in cells, which is controlled by a coordinated expression of specific molecules, notably proteins called cyclins. Cyclins are expressed in a particular sequence in specific cell cycle phases and are critical elements for cycle progression [1]. The conventional cell cycle phases include Gap 1 phase (G1), synthesis phase (S), Gap 2 phase (G2), and end in mitosis (M) where the cell splits into two daughter cells. In insects exist specific situations where cells can vary the plan and generate cells with different ploidy and/or DNA content, as in polytenic cells happening in dipteran salivary glands. Interestingly, the machinery that manages the conventional cell cycle is the same, participating in different options of the cell cycle [2-4].
One of the versions of a specialized cell cycle is the endoreplication. Endoreplication is a variant of the normal replicative cell cycle, in which cells increase their genomic DNA content without division. Endoreplication can enclose different options of the cell cycle; such as endocyles, re-replication, and endomitosis. The first one consists of repeated successions of S –G phases of all genetic material, without cell or nuclei division. In re-replication DNA synthesis is initiated multiple times at individual origins of replication within the same S phase, provoking site-specific replication of a unique sequence. In endomitosis an entry into mitosis occurs, the cells condense the chromosomes but do not dissociate them to daughter cells. Instead, they re-enter a similar phase to G1 and S phase starts again resulting in a multiple nuclei cell. [5]. The typical illustration of endoreplication is the generation of polytenic chromosomes in Drosophila salivary glands, but in many others, insects occur endoreplication. For example, in the beetle Tribolium castaneum larval stages, intestinal stem cells (ISC) conduct endoreplication for adult midgut polyploidic epithelium formation [6]. Also, in the flour moth Ephestia kuehniella, nuclei in Malpighian tubules and silk glands increase in size trough larvae instars. Even, in the last instar, larvae nuclei are polyploid with a high DNA content, provoking a branched nucleus. This polyploidy that results in branched nuclei, could be considered an adaptation because the distance between the nuclear area and the cytoplasmic zone is increased to permit traffic of molecules produced in high quantities [7].
Here, we discuss the cell cycle in the midgut development and the mechanisms conducting normal cell cycle into endoreplication. We describe endoreplication in the mosquito midgut as a fundamental part of its homeostasis. Finally, we also consider infection as a stressor when tissue undergoes damage and immune stimulation.
Cell cycle regulation during development
The regulation of the cell cycle depends on master mechanisms on several levels. Hormones that coordinate the cell cycle between different body parts and more fine mechanisms which act at the cellular/molecular level. Signaling pathways that detect environmental cues, including the hormones and chemicals, master molecules, as cyclins, kinases and phosphatases, that are synthesized and activated/desactivated or destroyed in an strict sequence in order to stop or induce the change from one to next cell cycle phase [8-11] The two main insect hormones, the Juvenile Hormone (JH) and the steroid hormone ecdysone and its active metabolites as 20- hydroxyecdysone (20-E) regulates development and reproductive maturation in insects.
JH is produced and released by the corpora allata (CA), endocrine glands connected brain [12], and their actions regulate reproduction, behavior, and diapause, between others. In Locusta migratoria, the allatectomy which leads to depletion of CA, inhibits de novo DNA synthesis. Application of Methoprene, a JH analog, restores JH-induced activities, including DNA synthesis and polyploidization in fat body cells. The mechanism involves methoprene- tolerant (Met), a JH receptor that induces transcriptional activation in minichromosome maintenance genes 4 and 7 (Mcm4, Mcm7), both genes involved in genome replication in fat body cells [13]. JH regulates the machinery of the cell cycle to originate polyploid cells supporting the massive synthesis of vitellogenin, one of the main proteins in eggs maturation. Also, another mechanism in fat body cell suggests that JH directs Cdk6 and E2f expression causing polyploid cells [14]. Methoprene is an analog of JH, used for insect control, that is not degraded and maintains its effect for a long time and is useful to evaluate JH actions. High methoprene concentrations avoid cell division in the midgut of larval-pupal transition, and it produces different grades of polyploidy in Ae. aegypti [10].
On the other hand, ecdysone signaling is an essential mediator in the switch between endocycle and site-specific endoreplication, trough the binding to the ecdysone receptor (EcR). At signaling pathways level, in epithelial follicle cells during oogenesis, Notch signaling is a mechanism that modulates endocycles, switching to site-specific gene amplification. Notch is down regulated while ecdysone signaling is activated and cooperate with Tramtrack (Tkt), a transcription factor that induces endocycle exit and entry in site-specific endoreplication [15]. Moreover, steroid hormone 20-hydroxyecdysone (20E) arrests in G1 phase the mosquito cell line C7-10 from Aedes albopicus and at the same time occurs down-regulation of cyclin A [16].
A “low-cost” strategy
Cell proliferation is a demanding process in material and energy terms. During oogenesis in Drosophila melanogaster, a stable nutritional status is important in ovariole to continue with the conventional proliferation program [17]. Also, in oogenesis, after mitotic proliferation occurs a transition to endoreplication that causes an amplification of specific DNA regions in which genes for essential situations are located, and that need to be mass-produced [18]. In nature, many organisms carry out endoreplication to provide a massive production of molecules. This strategy was eventually developed in cell types with high metabolic activity [19]. This hypothesis was proposed in the seeds of plants with endosperm. Endoreplication can enhance metabolic capacity because it occurs an increase in the gene copy numbers of genes related to metabolism, allowing synthesis of high quantities of relevant molecules which will be stored inside seed [20].
Replication, transcription, and translation have different energetic costs inside a cell. However, replication occurs at least one time, and transcription and translation are constant processes. An analysis in energetic terms from bacteria to eukaryotes indicates that the cost of a gene activity at the DNA, RNA, and protein level decline in relation with cell mass [21]. According to this, in specialized organs or cells, endoreplication would be, and actually it is, a very useful strategy to increase the cell and/or tissue size by polyploidy, generating cells efficient for specialized production during a short time avoiding mitotic cycles and spending less energy.
Cell cycle dynamics after infections
The hematophagous insect’s midgut is a monolayer of cells that can distend under the stretching of a large blood volume and absorb the nutrients from the blood meal. In mosquitoes, as in other insects, the midgut cells are differentiated in endocrinal, columnar, and regenerative cells.
Mosquitoes are the principal bloodfed arthropods responsible for transmitting critical diseases (vector-borne diseases) such as malaria, dengue, Zika, chikungunya, between others. In mosquitoes, there are data suggesting that after infections or immune challenges in specific tissues, including the midgut, cell cycle dynamics change [22], initiating regeneration and/or immunity functions. Cell cycle activation has been documented in Aedes albopictus midgut after chemical and bacterial damage [23]. These stimuli produced an increase in regenerative cells, allowing to the midgut to equilibrate the homeostasis. Besides, after viral infections and oxidative stress, cell proliferation has been tested in Aedes aegypti midgut [24].Evidence suggests that midgut infection is modulated by delayed or immediate activation of ISC to division. This activation is considered a strategy for tissue repair. However, polyploidization and growth by cell fusion also permit tissues to reach homeostasis with lower energy expenditure. It avoids the demand for an energetic process involving proliferation.
Metazoans maintain tissue size and morphology using cell division as a primary strategy to increase tissue size and repair. Although post-mitotic tissues can undergo compensatory cellular hypertrophy (CCI), consisting in hypertrophic growth without cell division, and this activation depends on the speed-up of endocycles rate, producing a cell with high DNA content and increased size to repair spaces in tissue after damage or apoptosis. Insulin/IGF (insulin- like growth factor)-like signaling pathway is involved in this strategy, intensifying endocycles rendering cells with increased cell size [25]. A hypothesis is that CCI strategy could be displayed during the insect immune response. In Anopheles albimanus tissues with a known relevant immune response and challenged with different microorganism showed a significant DNA synthesis [22,26,27]. This DNA synthesis has been attributed to an endoreplication process because any cell division has been observed. DNA synthesis was evidenced by BrdU incorporation in various tissues after immune challenge with Saccharomyces cerevisiae. Besides, after colchicine treatments an inhibition in BrdU incorporation occurs [26]. These results suggest that DNA synthesis is an active process caused by an immune challenge. Likewise, in Anopheles albimanus DNA synthesis after Plasmodium sp., challenge has been detected using BrdU incorporation [27]. This DNA synthesis is associated to an adaptative immune response, that in insects is known as immunological priming. Priming is defined as the ability to acquire a protective response to a pathogen as a consequence of previous exposure to the same organism. Such response has been probed in many invertebrates’ groups [28]. In An. albimanus, after a second exposure with Plasmodium berghei an increase in HNT, a protein that can regulate Notch pathway in proliferation and differentiation during development in follicular cells, [29] is detected. Similar results in Ae. aegypti after a second exposure to Dengue Virus [30] suggest an essential role of HNT in mosquito immunological priming and after oxidative stress [24]. The mechanism by which immunological priming occurs should be confirmed, but in mosquitoes, DNA synthesis and the expression of molecules related to endoreplication and stress-immunity response as antimicrobial peptides (AMP´s), are exciting results indicating that during the immune response the cell cycle is activated (Figure 1). In addittion, morphologic evidence suggests that after P. falciparum invasion and damage of mosquito midgut epithelium, cells undergo division and differentiation leading to regeneration [31]. However, the mechanism in epithelial repair after cell damage by Plasmodium gallinaceum invasion in Aedes aegypti midgut involves an actin cone that mediates cells displacement without cell division evidence [32].
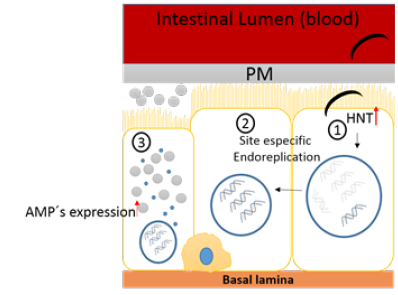
Figure 1: DNA synthesis induction in the mosquito midgut, during immunological priming. In an Anopheles midgut columnar cell challenged with Plasmodium berghei HNT activity is activated (1), leading to amplification of DNA specific regions (colored DNA double helix). Among the amplified genes are stress-immunity response molecules and antimicrobial peptides (AMP´s) (site-specific endoreplication) (2). In a second Plasmodium challenge (3), transcription of amplified genes occurs rapidly leading to an increase in expression of immune molecules which respond against Plasmodium. The light-yellow cell adjacent to basal lamina corresponds to a regenerative cell. Endocrine cells were omitted. PM.- Peritrophic matrix. AMPs. - Antimicrobial peptides. HNT. - Hindsight protein.
Conclusion
In insects, particularly in dipterans as flyes and mosquitoes, cells needing the massive synthesis of molecules, required during reproduction and other tasks, carry-out the amplification of DNA segments, bearing genes specific to be used as templates for extensive transcription of messengers that will be used to produce useful molecules. This dynamic response is “low-cost” compared with other strategies, including the cell division, which needs a lot of material, energy, and time. There is increasing evidence that in mosquitoes, this strategy can be deployed to face immune challenges, and to prepare the individuals against repetitive pathogens assaults. These actions lead the mosquitoes to a “primed” status, in which the insect will be resistant to specific infections. Although there is a lot of molecules and mechanisms that are unknown in this critical group of insects. This is an exciting field that should be explored; to understand the mosquito-pathogens relations, and to design creative strategies to interfere with disease transmission.
Acknowledgements
FCH received the “Estimulo para la Investigación Biomédica de la Fundación Miguel Alemán, 2018”.
Conflict of Interest
Authors declare that do not have any conflict of interest.
References
- Breeden LL (2000) Cyclin transcription: Timing is everything. Current Biology 10(16): 586-588.
- Follette PJ, Duronio RJ, Farrell PHO (2009) Fluctuations in Cyclin E levels are required for multiple rounds of endocycle S phase in Drosophila. Curr. Biology 8(4): 235-238.
- Huang J, Raff JW (1999) The disappearance of cyclin B at the end of mitosis is regulated spatially in Drosophila cells. The EMBO Journal 18(8): 2184-2195.
- Veylder L De, Larkin JC, Schnittger A (2011) Molecular control and function of endoreplication in development and physiology. Trends in Plant Science 16(11): 624-634.
- Lee HO, Davidson JM, Duronio RJ (2009) Endoreplication: polyploidy with purpose. Genes & Development 23(21): 2461-2477.
- Parthasarathy R, Palli SR (2008) Proliferation and Differentiation of Intestinal Stem Cells During Metamorphosis of the Red Flour Beetle, Tribolium castaneum. Developmental dynamics 237(4): 893-908.
- Buntrock L, Marec F, Krueger S, Traut W (2012) Organ growth without cell division: somatic polyploidy in a moth, Ephestia kuehniella. Genome 55(11): 755-763.
- Ables ET, Grace H Hwang, Danielle S Finger, Taylor DHinnant, Daniela Drummond Barbosa (2016) Ecdysone-Responsive Genes Regulating Drosophila Oogenesis. Genes Genomes and Genetics 6(8): 2629-2642.
- Sprenger F, Yakubovich N, Farrell PHO (1997) S-phase function of Drosophila cyclin A and its downregulation in G1 phase. Current Biology 7(7): 488-499.
- Nishiura JT, Ho P, Ray K (2003) Methoprene Interferes with Mosquito Midgut Remodeling During Metamorphosis. Journal of medical entomology 40(4): 498-507.
- Knoblich JA, Sauer K, Jones L, Richardson H, Saint R, et al. (1994) Cyclin E Controls S Phase Progression and Its Down-Regulation during Drosophila Embtyogenesis Is Required for the Arrest of Cell Proliferation. Cell 77(1): 107-120.
- Noriega FG (2014) Juvenile Hormone Biosynthesis in Insects: What Is New, What Do We Know, and What Questions Remain? International Scholarly Research Notices 2014: 967361.
- Guo W, Wu Z, Song J, Jiang F, Wang Z, et al (2014) Juvenile Hormone-Receptor Complex Acts on Mcm4 and Mcm7 to Promote Polyploidy and Vitellogenesis in the Migratory Locust. Plos Genetics 10(10): e1004702.
- Wu Z, Guo W, Yang L, He Q, Zhou S (2018) Juvenile hormone promotes locust fat body cell polyploidization and vitellogenesis by activating the transcription of Cdk6 and E2f1. Insect Biochemistry and Molecular Biology 102(7): 1-10.
- Sun J, Laila Smith, Alexander Armento, Wu-Min Deng (2008) Regulation of the endocycle/gene amplifi cation switch by Notch and ecdysone signaling. Journal of cell biology 182(5): 885-896.
- Fallon AM, Gerenday A (2010) Ecdysone and the cell cycle: Investigations in a mosquito cell line. Journal of Insect Physiology 56(10): 1396- 1401.
- Laws KM, Drummond Barbosa D (2016) AMP-activated protein kinase has diet-dependent and -independent roles in Drosophila oogenesis. Developmental Biology 420(1): 90-99.
- Royzman I, Orr weaver TL (1998) S phase and differential DNA replication during Drosophila oogenesis. Genes to cells 3(12): 767-776.
- Edgar BA, Orr weaver TL (2001) Endoreplication Cell Cycles: More for Less. Cell 105(3): 297-306.
- Inz, D, De Veylder L (2006) Cell Cycle Regulation in Plant Development. Annual Review of Genetics 40: 77-105.
- Lynch M, Marinov GK (2015) The bioenergetic costs of a gene. Proceedings of the National Academy of Sciences of the United States of America 112(51): 15690-15695.
- Hernández Martinez S, Barradas bautista D, Rodríguez MH (2013) Diferential dna synthesis in Anopheles albimanus tissues induced by immune challenge with different microorganisms. Archives of Insect Biochemistry and Physiology 84(1): 1-14.
- Janeh M, Osman D, Kambris Z (2017) Damage-Induced Cell Regeneration in the Midgut of Aedes albopictus Mosquitoes. Scientific Reports, 7: 1-10.
- Taracena ML, Bottino Rojas V, Talyuli OAC, Walter Nuno AB, Oliveira JHM (2018) Regulation of midgut cell proliferation impacts Aedes aegypti susceptibility to dengue virus. PLOS Neglected Tropical Diseases 12(5): e0006498.
- Tamori Y, Deng W (2014) Compensatory cellular hypertrophy: the other strategy for tissue homeostasis. Trends in Cell Biology, 24(4): 230-237.
- Hernández Martinez S, Román Martínez U, Martínez Barnetche J, Garrido E, Rodríguez MH, et al. (2006) Induction of DNA Synthesis in Anopheles albimanus Tissue Cultures in Response to a Saccharomyces cerevisiae Challenge. Archives of Insect Biochemistry and Physiology, 63(4): 147-158.
- Contreras garduño J, Rodríguez MC, Hernández-Martínez S, Martínez-Barnetche J, Alvarado-Delgado A, et al. (2015) Plasmodium berghei induced priming in Anopheles albimanus independently of bacterial co-infection. Developmental and Comparative Immunology 52(2): 172-181.
- Kurtz J, Milutinovi B (2016) Immune memory in invertebrates. Seminars in Immunology 28(4): 328-342.
- Sun J, Deng W (2007) Hindsight Mediates the Role of Notch in Suppressing Hedgehog Signaling and Cell Proliferation. Developmental Cell, 12(3): 431-442.
- Serrato salas J, Izquierdo-Sánchez J, Argüello M, Conde R, et al. (2018) Aedes aegypti antiviral adaptive response against DENV-2. Developmental and Comparative Immunology 84: 28-36.
- Baton LA, Ranford Cartwright LC (2011) Morphological evidence for proliferative regeneration of the Anopheles stephensi midgut epithelium following Plasmodium falciparum ookinete invasion. Journal of invertebrate pathology 96(3): 244-254.
- Gupta L, Kumar S, Han YS, Pimenta PF, Barillas Mury C (2005) Midgut epithelial responses of different mosquito – Plasmodium combinations: The actin cone zipper repair mechanism in Aedes aegypti. PNAS 102(11): 4010-4015.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.