Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
The Evolution of the Nervous System: Invertebrates
vs. Vertebrates a useful Instrument and Model to
Research New Pharmacological Strategies in some
Human Neurodegenerative Conditions
*Corresponding author: Safila Naveed, 4Department of Eastern Medicine, Government College University, Pakistan
Received: August 20, 2019; Published: October 01, 2019
DOI: 10.34297/AJBSR.2019.05.000960
Abstract
Nervous System Functions, Evolutive Pattern, and Relationship with Some Neurodegenerative Condition:
The nervous system directs and coordinates our movements. It receives stimuli from the environment around us and from all our internal organs.
It interprets these stimuli and elaborates responses, which are transmitted to muscles or glands. To compare the nervous systems of invertebrates
to vertebrates and between the various vertebrates.
Can be a useful instrument in field of neuroscience, molecular biology, forensic science, and pharmacology, regenerative medicine.
Why in example some neuronal circuits in superior vertebrates cross themselves like decussatio pyramid or in optical chiasm?
And why in brain vs spinal cord grey matter and white matter are inverse (opposite)?
And this fact makes possible to verify relationship between degenerative pathology of cortex vs spinal cord under an evolutive approach.
And this can be related also to the “brain and spinal cord wasting system” functionality?
Is universally known that different neurodegenerative disease involved different CNS parts: cortex (DA), PD (basal nuclei), ALS spinal cord
preferentially.
And this is an interesting fact: a same organ (CNS) but whit different neuronal sensibility to the damages like neurodegeneration.
Is universally known that in superior vertebrates AD is a common disease in animals whit medium -high cognitive functions (frontal cortical and
other) and not only a human condition
An evolutionary approach makes possible to translate to applied research crucial information to better Explore new therapeutic strategies for some
neurodegenerative pathology.
Keywords: Nervous Systems; Vertebrates; Invertebrates; Neuroscience; Evolutive; Research; Neurodegenerative; Forensic Sciences; Pharmacology
Research; Regenerative Medicine
Introduction
Is possible to start this work with a question: observing various
animals is possible to see the same incidence of neurodegenerative
disease?
DA is not only a human pathology, other species present this
condition (cats, dogs and other).
Obviously is superior vertebrate’s whit high cortical – cognitive
evolution.
If this function is less physiologically – anatomically developed
is difficult that this animal can show DA.
Is a Paradox, but in evolutive pattern of superior vertebrates
something goes wrong?
The different vulnerability of CNS neurons in the different place
of the brain or spinal cord seem to tell us that in evolution the
new structure added to the oldest are more vulnerable.
For this reason, is crucial to set the neurodegenerative disease
under an evolutionary
Approach.
A more complex nervous system (invertebrates vs vertebrates)
create a very different organ with advantages but also
disadvantages.
From university lessons, adapted: The Evolution of the Nervous
System (by Gaber Ibrahim)
In the lower multicellular animals, such as porifers or sponges,
there is no rudiment of nerves. We begin to see neurons, cells that
conduct nerve stimuli, in coelenterates. In cnidarian polyps these
cells appear scattered throughout the body, forming a network
without much organization. There is no nerve center in these
animals that runs this network. Each external stimulus acting on
a point on the body is accompanied by a merely local response,
determining a nerve impulse that propagates with decreasing
intensity as it moves away from the stimulus’s starting point.
Cnidaria have a diffuse nervous system.
Cnidarian Phylum (corals, anemones, hydras and
jellyfish)
The more primitive Porifers (sponges) do not have a nervous
system. In Cnidaria, there is a disordered network of neurons.
And if a nerve pulse is triggered in one of them, it is transmitted
to all cells that communicate with it through synapses, and from
these to others, resulting in poorly elaborated responses - such as
“pulsating” movements in living water when it’s swimming. It is
the most primitive type of nervous system, called Diffuse Nervous
System.
In Flatworm Worms (such as planar worms, for example)
Neurons associate together to form nerve threads attached
to some structures - the nerve ganglia in the head. These ganglia
already represent precarious nerve centers in coordinating body
activities. In each ganglion there is a higher concentration of
neurons.
The ganglionic nervous system begins to perfect in the annelids.
In them, there is a larger conglomeration of neurons in the head,
forming the cerebroid ganglia, which play a primitive brain role in
commanding the other ganglia. From the cerebroid ganglia arise the
periesophageal ganglia, which relate to a double ventral ganglionic
nerve chain. Along this chain there are a pair of ganglia for each
body segment. These ganglia also have marked autonomy over the
specific activities of the surrounding body area.
In Annelids
Notwithstanding the presence of cerebroid ganglia, the pairs of
ganglia along the ventral nerve chain have a great deal of autonomy,
so a worm, even after being cut in half, continues to move the two
pieces apart.
The cerebroid ganglia are even more developed in arthropods,
especially insects.
In bilateral symmetry invertebrates (Platelminths,
Nematelmints, Annelids, Molluscs and Arthropods) the nervous
system is in the ventral region of the body and is organized as one
or more longitudinal nerve cords presenting two or more nerve
ganglia, whether functioning as command centers along its length.
In the possessors of many nerve ganglia, those in the anterior
region-cerebroid ganglia are more developed and function as
a rudimentary brain that controls the other ganglia. This type of
nervous organization is called the ganglionic nervous system.
In Mollusca
The nervous system is centralized and ganglionic, with three
parts of nerve ganglia from which nerves go to different parts of the
body. Sensory, visual, tactile, chemoreceptor and balance structures
are present. The cephalopods have a large cerebroid ganglion that
resembles the brain of vertebrates.
In gastropods
The nervous system consists of a set of ganglia and cords that are
distributed throughout the body and innervate the different organs.
The set of sensory organs comprises eyes, tentacles, asphradium and
statocysts. The eyes, in the most primitive forms, are located at the
ends of the tentacles and consist of simple depressions containing
pigment and photoreceptor cells. In more advanced gastropods,
depression closes, and a cornea and a lens are distinguished. The
tentacles have eyes and tactile and chemoreceptor cells. Statocysts
are important sensory cells for balance. Available only in species
with gills, appears to function as an olfactory and chemoreceptor
organ.
Take a close note: The ganglionic nervous system, which
characterizes invertebrates, has its double chain of lymph nodes
arranged ventrally in the animal, that is, running along the
ventral surface of the body. This system is in stark contrast to the
vertebrate brain-spinal nervous system that we will see next. The
cerebrospinal nervous system is in the dorsal position, descending
from the head along the back of the animal [1].
The Cerebrospinal Nervous System
In vertebrates (fish, amphibians, reptiles, birds and mammals),
the nervous system is well developed and is classified as a cerebrospinal nervous system. It is made up of a “thirst” - the CNS
(central nervous system) - and a network of nerves that break out
and distribute throughout the body - the peripheral nervous system.
Vertebrates
4.1.1. The Central nervous system: The CNS is formed by the
brain and spinal cord. The brain, in turn, comprises the following
portions: brain, cerebellum, protuberance (pons or menencephalon)
and bulb.
In the lower vertebrates, from fish to birds, the cerebral
hemispheres have a smooth surface. Such animals are considered
diencephalon (smooth brain). In mammals, however, grooves
and circumvolutions appear, giving the brain a surface full of
undulations. For this reason, mammals are called gyrencephalon
(brain with turns or curves). This transformation brought a great
advantage for mammals: At the same volume, a circumvoluted
brain has a considerably larger surface than if it had smooth
hemispheres. As it is on the surface of the brain (cerebral cortex,
with gray matter) that lie the bodies of neurons, the more grooves
and convolutions the brain has, the larger its cortex, the larger the
number of neurons, and thus the more efficient and improved it is.
The gray matter is placed on the surface of the brain and is
where the bodies of neurons accumulate. It is in them that the
information is stored, the senses are perceived, the data obtained
from external stimuli are “processed”. Also, from the neurons depart
the orders for muscle contractions or for glandular secretions
etc. This superficial area is the cerebral cortex. It has the greatest
importance in the degree of development of a species.
The cerebral cortex is all divided into zones, like a map. Each
area (some small, some large) represents a nerve center. Nervous
centers are numerous throughout the brain, such as the centers
of sight, hearing, smell, taste, pain, hunger, cough, tickling, anger,
motor coordination (this is very wide and subdivides into areas
corresponding to the various points of the body), the visual
association for reading, in addition to the centers of respiratory,
cardiac regulation, the thermoregulatory center, etc. The cortex is,
as it turns out, the “seat” of control of conscious and unconscious
acts as well as intelligence.
The brain of a crocodile is, of course, larger than a brain of a
mouse. However, the crocodile, as a reptile, is lissencephalon,
while the mouse, as a mammal, is a gyrencephalon. Therefore, the
extension of the cerebral cortex of the mouse is larger than that of
the crocodile, justifying greater rodent intelligence. That is why, in
circuses, animal shows predominantly exhibit mammals.
In the deepest region of the brain lies the white mass. In it,
there are practically no bodies of neurons, but only their branches
(dentites and axons).
The cerebellum, pons, and bulb are also very important
because they enclose nerve centers that regulate various functions
of relevant role. Breath and temperature controls are in the bulb.
Control of body balance is in the cerebellum.
Aside from the brain, the remainder of the CNS consists of the
spinal cord (or spinal cord). It is a long cord of nervous structure
that runs along the dorsum inside the spinal canal. It is therefore
protected to its full extent by the spine. In spinal cord the gray mass
(as opposed to the brain) is in the center and the white mass in the
periphery.
White matter is buried deep in the brain and the gray matter is
mostly found on the brain’s - cortex.
The spinal cord that transmits nerve impulses to and from the
rest of the body, has an opposite arrangement:
gray matter at its core with insulating white matter on the
outside.
gray matter at its core with insulating white matter on the
outside.
These tracts transmit the electrical signals that the brain
neurons, to communicate.
They’re wrapped in a fatty- layer named myelin, this insulates
axons, allows them to conduct signals in very quickly way, much
like rubber insulation does for electrical wires
The type of fat in myelin makes it seem white.
Gray matter is mostly neuron cell bodies and non-neuron brain
the cells named glial cells, that provide nutrients and energy to
the neurons.
They help in the transport of the glucose into the brain, clean
the brain of excess chemicals and may even affect the intensity
of the neuron’ s- communications systems.
In central nervous system there are a mix of cell types present
in both gray and white matter.
a. Gray Matter Contains:
• Neurons
• Axon tracts
• Glial cells
• Capillary blood vessels
• Neuropil-mix of dendrites, un-myelinated axons, glia
b. White Matter contains:
• Oligodendrocytes-glial cells which produce myelin
• Astrocytes
Function of Gray Matter
a) Gray matter-heavy- brain regions include those that
control muscular /sensory activity.
b) The outer layer of the brain, the cerebral cortex, consists
of columns of gray matter neurons, with white matter located
underneath.
c) This area is essential to many facets of higher learning
functions, attention, memory, and thought.
d) The cerebellum is essential for motor control/
coordination/ and precision of movements
Function of White Matter
e) Neuron-rich brain regions join themselves by the rich
veins of axonal connections contained within white matter
f) The white fatty myelin is essential to its function – myelin
insulates axons, letting the signal within travel far faster, this
make possible the nerve cell function: essential to normal
motor and sensory function (Figure 1 & 2
).
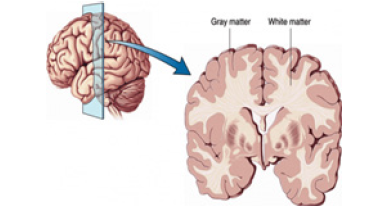
Figure 1:Brain grey matter outside, white matter inside.
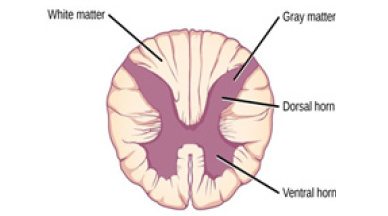
Figure 2:Spinal cord grey matter-white matter.

Table 1:
The brain and spinal cord are fully protected by bone structures
(the skull and spine) and by three outer membranes or meninges:
a. Dura Mater - outermost, thick and fibrous;
b. Arachnoid - has a vascularization that resembles a spider
web;
c. Pia Mater - the most internal, thin and adherent to the
CNS.
Below the arachnoid is the cerebrospinal fluid or cerebrospinal
fluid, which has a protective function, involving the entire CNS.
The spinal cord does not fully occupy the vertebral canal. It
ends at about the level of the 1st or 2nd lumbar vertebra. Spinal
anesthesia, which worries so many people, offers no danger of
traumatizing the spinal cord because it is made with a needle that
penetrates the spinal canal below the 2nd lumbar vertebra. Below
this level is only one very thin fibrous cord - the filum Terminable -
that attaches the lower end of the medulla to the coccyx. Thus, the
marrow should always be stretched.
On its way, the spinal cord emits the spinal nerves, always in
pairs. And you may notice that these nerves are closely related to
gray matter
Many reflex acts are controlled directly by the spinal cord
without brain interference. But in most cases, nerve stimuli
reaching this organ are then transmitted to the brain, first reaching
the diencephalon (region covering the hypothalamus) and then
radiating to the most varied areas of the brain.
Is possible to verify that during vertebrate’s evolution increased
brain volume vs inferior vertebrates and this was responsible of
the opposite architecture of brain vs spinal cord related white- grey
matter anatomy.
The cortex is more focused in the new superior cognitive
function’s vs spinal cord
Whit more specialization in condition of the stimuli [2].
The Peripheral Nervous System
The PNS consists of the internal network of nerves that depart
from the CNS and are distributed throughout the body (motor
nerves) and from nerves that come from all areas of the body and
converge on the CNS (sensory nerves). Of course, there are mixed
nerves whose characteristics include those of all types mentioned
above, that is, they carry all orders of the CNS to the various points
of the body and at the same time transmit the sensory perceptions
of those same points to the CNS.
We can then say that the PNS (peripheral nervous system)
comprises all the nerves in our body. Many of these nerves act on the
will of the individual, revealing voluntary action. These voluntary
action motor nerves, along with the sensory nerves (which allow
us to see, hear, feel pain, smell, taste, heat or cold etc.), offer the
individual the possibility to relate to the environment. Therefore,
they form what we may call the nervous system of relationship life.
This system contrasts with another large number of nerves that act
without the individual’s conscience or will, regulating the activity of
numerous organs such as the heart, stomach, intestines, diaphragm
movements, salivary gland secretions, the pupil diameter etc.
These involuntarily acting nerves, which work without one even
suspecting, together form the autonomic nervous system or the
vegetative life nervous system.
There are lesions that destroy areas of the CNS, completely
nullifying the nervous system acting on the relationship life
nervous system but leaving the nervous system of the vegetative
life intact. When this occurs, the person becomes unrelated to the
world around him and goes on to live an extremely vegetative life (the organs work well, but the individual seems to feel nothing or
respond to external stimuli).
It is common to call the nervous system of life a somatic nervous
system relation (from the Greek soma, “body”), which does not
seem very logical to us, since the autonomic nervous system, acting
on the various parts of the body, is, consequently also somatic.
The nervous system of relationship life comprises nerves that
originate directly in the brain (particularly, the brain, cerebellum,
pons or bulge, and, more numerously, the bulb) and nerves that
originate in the spinal cord. We then distinguished cranial and
spinal nerves, respectively.
Cranial nerves are those that are born directly from the brain.
In mammals they number 12 pairs (in other vertebrates there are
only 10 pairs). Some are sensitive; others, engines; still others are
mixed. All are cataloged by numbers. Often, a pair is referred to by
its number, not by its name.
Thus, it is mandatory to know the 12 pairs of cranial nerves by
their order numbers:
1. Olfactory (sensitive): It transmits to the brain the impulses
that give the perception of smell.
2. Optical (sensitive): Brings to the brain the impulses that
provide the visual sensations (Figure 3
).
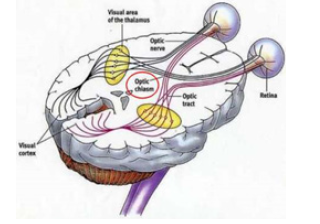
Figure 3:Human optical chiasm.
3. Common Eye Engine or Oculomotor (engine): Moves the
eyes up, down and in (nose direction).
4. Pathetic or Trochlear (motor): Makes the eyes rotate
circularly.
5. Trigeminal (mixed): Perceives sensations of the face and
acts on the muscles of the mimic.
6. Outboard Eye Engine or Abdulent (motor): Moves the
eyes outward.
7. Facial (mixed): It transmits the skin sensations of the face
and acts in mimicry.
8. Acoustic or Atrio-cochlear (sensitive): One of its branches
leads to the brain impulses that will give sound perceptions.
The other leads to the cerebellum impulses responsible for the
notion of body balance.
9. Glossopharyngeal (mixed): Transmits the impulses that
give the perception of taste and moves the tongue.
10. Pneumogastric or Vagus (mixed): It acts on the
thoracic and abdominal organs and is the main nerve of the
parasympathetic system.
11. Spinal, Spinal or Accessory (motor): Acts on the shoulder
muscles (shoulder slapping of the naughty).
Hypoglossus (motor):
Helps glossopharyngeal in tongue movement.
They all act on organs and muscles from head to shoulder. Only
the pneumogastric or vagus goes into the body and innervates the
viscera, such as the heart, stomach, intestines and other organs.
In fact, this is the only cranial pair that has involuntary action,
therefore belonging to the autonomic nervous system.
The spinal nerves are all born from the spinal cord, but they go
to different parts of the body, such as arms, trunks and legs. They
comprise 31 pairs and are all mixed, that is, they transmit sensations
of the skin and organs to the spinal cord, as they transmit its motor
orders to the muscles.
Each spinal nerve contains sensory fibers, which bring to the
medulla sensory perceptions of a region of the body, and motor
fibers, which carry motor stimuli from the medulla to these regions.
The spinal nerves emerge from the medulla through two roots -
anterior root and posterior root - which join just below to form the
nerve itself.
Posterior roots (with sensory fibers) are afferent to the medulla,
as they conduct the stimulus towards it. The anterior roots (with
motor fibers) are efferent in relation to the medulla, because they
carry stimuli that move away from it.
To its greatest extent, therefore, each spinal nerve encloses
sensory and motor fibers and proceeds as a “two-way road”. From
the sensory fibers come the perceptual stimuli and from the motor
fibers the command commands.
Transition (or association) neurons can make the connection
between a sensory neuron and a motor neuron:
A. on the same side and at the same level as the gray matter
of the medulla;
B. opposite but at the same level in the medulla (horizontal
cross-association);
C. on the opposite -side and at another level in the medulla
(vertical cross association);
D. on the same side and at another level (uncrossed vertical
association).
Surely you have ever touched a finger harder than you expected
on the tip of a needle. And he withdrew his finger abruptly, so quickly that it would not be possible or consciously for him. This fact is an
example of arc reflex. The reflex arc is the immediate response to
arousal of a nerve without the interference of the individual’s will
(and sometimes even consciousness).
In the above example, the stimulus ran through the sensory
fibers of a spinal nerve, bypassed the gray matter of the spinal cord
by the association neuron, and returned through the motor fibers of
the spinal nerve, reaching the muscles of the arm and hand, causing
them to contract. and remove the finger from the tip of the needle.
Many reflexes are by medullary mechanism only. The rotulian
or patellar reflex, which the doctor investigates a small blow to
the rotulian tendon (knee), denotes through the sudden response
of the musculature, involuntarily kicking the air, that the spinal
nerves of this region, as well as the medulla, are perfect and in good
working order.
But some reflexes are more complex and involve stimuli that go
to and return to the cerebral cortex bringing orders to the marrow
From the analysis of the figure above, you can see:
At the level of the bulb, stimuli from one side of the body
transfer to the opposite -side of the brain, just as motors coming
from one cerebral hemisphere cross at the level of the bulb or
medulla. Across the body in a reflex involving the medulla and the
brain, the sensory stimulus moves back and forth at the level of the
bulb, but the motor response coming from the brain only reverts to
the primitive side at the level of the medulla (Figure 4
) [3].
Literature
a. Quan Wen et al. [7]
“A ubiquitous feature of the vertebrate anatomy is the
segregation of the brain into white and gray matter. If evolution
maximized brain- functionality, what is the reason for such
segregation? To answer this, we posit that brain functionality
requires high inter-connectivity and short conduction delays.
Based on this assumption we re-searched for the optimal brain
architecture by comparing different candidate designs. We found
that the optimal design depends on the number of neurons, interneuronal
connectivity, and axon diameter. the requirement to
connect neurons with many fast axons drives the segregation of the
brain into white and gray matter. These results provide a possible
explanation for the structure of various regions of the vertebrate
brain, such as the mammalian neo-cortex and neo-striatum, the
avian tele-encephalon, and the spinal cord” [7].
b. Carlos Matute et al. [8]
“The phylogenetic enlargement of cerebral cortex culminating
in the human brain imposed greater communication needs that
have been met by the massive expansion of WM (white matter), as
opposed to the GM (grey matter), exclusively contains axons and
their glial cell partners; absent from WM are neuronal cell bodies,
dendrites and conventional synaptic structures. Glial cells in WM
are unique. WM astrocytes have especially long, highly discrete
processes, which have led to their designation as ‘fibrous’ astrocytes.
Oligo-centrocytes, which make and sustain myelin, predominate in
WM, although their density varies regionally as a function of the
percentage of axons that are myelinated in a given tract (e.g. 100%
in optic nerve to fewer in corpus callosum). Myelin consists of
tightly wrapped oligo-dendrocytic processes that surround larger
diameter axons and mediate saltatory action potential conduction,
which increases conduction velocity by at least 50-fold compared
with un-myelinatedfibres of similar diameter” [8].
c. Giulio Srubek Tomassy et al. [9]
“The evolutionary success of the vertebrate NS is largely due to
a unique structural feature - the myelin sheath, a fatty envelope that
surrounds the axons of neurons. By increasing the speed by which
electrical signals travel along axons, myelin facilitates neuronalcommunication
between the distant regions of the nervous system
Myelin evolution.
As animals populated different and more challenging
environments, the rapid conduction of the nerve impulses must
have constituted a great adaptive advantage, enhancing chances of
survival for both predators and prey. Neuronal impulse conduction
is modeled as a flow of ions through a hollow cylinder. In this
model, 2 physical parameters of the cylinder critically affect the
speed of ion flow: axial resistance and capacitance of the surface.
Through alterations in either or both 2 parameters, different
evolutionary strategies achieved the same goal – increasing the
conduction velocity of neuronal signals to make “faster” NS. In
some species, axonal diameter was increased in order to decrease
the axonal internal resistance and thus speed up signal conduction,
an adaptation which resulted in the ‘giant axons’ of many invertebrates;
in vertebrates, development of the myelin sheath
increased the axial resistance of the axonal surface in addition to
reducing its capacitance.
Robust evidence exists for the evolutionary- advantage that
myelin provides. Myelin repeatedly emerges among species that
are phylo-genetically distally unrelated; myelin-like structures are
found even in some in-vertebrates, like members of the subphylum
Crustacea (some decapods and copepods) and phylum Annelida
(e.g. earthworms). Among myelinated invertebrate species, the
structure of the myelin envelope can vary considerably from a loose
arrangement of lamellae (e.g. in some decapods) to a “vertebratelike”
compact architecture comprising tightly associated layers
(earthworms). Bio-chemical properties, such as content and ratio of
proteins and lipids within the myelin envelope, can vary among invertebrate
species. In an even more distant example of evolutionary
diversity, myelin-like structures with no glial origin were recently
described in the nervous system of the copepod Bestiolinasimilis.
Myelin found around the axons of gnathostome vertebrates is
more homogeneous and structurally similar, even between CNS and
PNS, with fine differences observed only at higher magnification.
Myelin is present in all vertebrates, from cartilaginous fishes to
mammals, with the exception of the class Agnatha, jawless fish; for
this reason, it has been hypothesized that appearance of myelin
was concomitant with the appearance of a hinged jaw and that first
myelinated gnathostomes may have been the placoderms (among
the first jawed fish), whereas other jawless fish (e.g. ostracoderms)
may have not been myelinated.
Whether loose or compact, produced by Schwann cells, oligodendrocytes,
and by yet-to-be-defined mechanisms, myelin is a
perfect example of convergent evolution. By increasing the speed of
impulse conduction, myelin certainly contributed to the expansion
of the vertebrate brain and to the emergence of complex- plastic
behaviors “[9].
d. Michel A Hofman [10]
“The most obvious problem imposed by large brains is
increasing distances among the neuronal somata of functionally
related regions and the inevitable lengthening of their essential
communication lines, the axons. the axonal length and volume
increase much more rapidly than the number of neurons. a
proportional increase of neurons and connections would inevitably
lead to a rapid increase of synaptic path length, defined as the
average number of mono-synaptic connections in the shortest path
between two neurons So that the path length can be maintained short cut connections can be inserted, resulting in small-world- and
scale-free-type networks.
Although such a solution can effectively decrease path length
within the neo-cortex, the increased lengths of the axons and the
associated increased travel time of the action potentials still pose
serious problems. As compensation for these excessive delays, axon
caliber and myelination should be increased .An indication that
larger brains deploy both more shortcuts (long-range connections)
and larger-caliber axons is that the volume of the white matter
increased at 4/3 power of the volume of gray matter during the
course of evolution. Although the white matter occupies only 6% of
the neo-cortical volume in hedgehogs, it exceeds 40% in humans”
[10].
e. Cassandra Sampaio-Baptista et al. [11]
“Recent evidence suggests that oligo-dendrocytes can form
compact myelin sheaths even in the absence of molecular axonal
cues, and that sheath length depends not on properties of the fiber
but on the regional origin of the oligo-dendrocyte (brain versus
spinal cord “[11].
f. Marc R Freeman et al. [12]
“III. Evolution of Brain Complexity: More and Diversified
Glia Is, Evidently, Better
In-vertebrate glia carry out many functions that are analogous
to their vertebrate counterparts. The Drosophila nervous system
comprises about 105 neurons compared to 85 × 106 neurons in
the human brain. Glia make up about 15% of the C. elegans and
Drosophila nervous systems, but estimates range from 50%–90% of
cells in the human brain, implying that greater glial numbers were
essential for achieving increased brain complexity. The increased
size of the brain required new mechanisms for proliferation and
expansion of glial pool size and long-range conduction across
white matter tracts. Beyond just increasing numbers, glia may
also have acquired enhanced functions and diversity. Cell-intrinsic
morphological and functional differences have been observed
within mammals between mouse and human astrocytes. Other
examples of enhanced glial functions are below
Strategies to Enhance Nerve Conduction
Selective pressure for more rapid conduction of the nervous
impulse, e.g., in escape or attack behaviors, increasing brain
complexity, etc., resulted in 2 types of solutions: decreasing
longitudinal resistance or increasing capacitance of axons.
Invertebrates have unsheathing cells but generally lack myelin.
Exceptions are earthworms, copepods, and some crustacean
nerves, but myelin and organized white matter tract, as such, are
generally found only in vertebrates above the jawless fishes. In
non-myelinated axons, velocity of the action potential is directly
proportional to the axon diameter. The major conduction speed
augmentation strategy in in-vertebrates is reducing longitudinal
resistance by increasing the diameter of axons. Prime examples
of this are found in cephalopods that accommodate a very large
diameter axon or the Drosophila giant fiber, which drives the
escape response.
Vertebrates have other constraints that place limits on using this
strategy, including limiting bony structures, greater size requiring
longer axonal lengths in the CNS and PNS, and with increasing brain
complexity there is the need to pack many more axons in each
space. The solution for accommodating many small-diameter axons
is to reduce the effective capacitance and increase the effective
membrane resistance, which is achieved by providing a layer of
insulation, which is achieved with myelination. Myelin sheathes
also organize sodium channels into clusters (nodes of Ranvier) for
saltatory (jumping) conduction. For an axon of equivalent diameter,
myelin can increase the velocity of nervous impulse conduction by
50- to 100-fold. It should also be noted that oligo-dendrocytes carry
out other functions in support of axon integrity, likely an adaptation
brought about to deal with energy and trophic demands of the extraordinarily
long fast-firing axons found in many higher organisms.
a recent study showed that deficiency of a lactate transporter in
oligo-dendrocytes led to axono-pathy and degeneration “[12].
g. Suzana Herculano-Houzel et al. [13]
“Scaling of ratios of neurons over the rest of brain
The spinal cord and brain-stem are the portions of the CNS that
are most directly related to the regulation of bodily functions, and
thus could be expected to scale in close relationship to the scaling
of body physiology in its various aspects. Neurons in the cerebral
cortex and the cerebellum, in contrast, are believed to add a whole
new level of elaboration to the processing of information relayed
from the body and back to it through associative processing,
endowing animals with more refined and flexible behavioral
repertoires.
In the absence of data on numbers of neurons and volumetric
data for the spinal cord, the ratio of cortical volume over the
volume of the medulla has been proposed as a value that should
predict cognitive capacity in a manner that is not biased by body
mass Variations in this ratio across primate species indeed were
well correlated with available behavioral data, but so were brain
size, relative cortical volume and encephalization quotient . that
comparison assumed that the volumes of the cerebral cortex and
of the medulla are good proxies for numbers of neurons in the
structures, whereas we have shown that this is not the case across
clades. the ratio between numbers of neurons in the cerebral cortex
and in the brainstem, or spinal cord, might provide a good estimate
of how cortical processing capacity scales beyond body-related
information processing across species.
Across primate species, we found that numbers of neurons
in the spinal cord are linearly related to the length of the spinal
cord, not body mass .the cerebral cortex gains neurons as a power function of numbers of neurons in the spinal cord with exponent
2, even though the mass of the cerebral cortex (including white
matter) scales only slightly faster than the mass of the spinal cord,
as a power function of exponent 1.
Un-fortunately, data on total numbers of neurons in the spinal
cord that can be compared to numbers of neurons in the brain
are only available for primates. we found in that study that the
number of neurons in the ensemble of brainstem, diencephalon and
striatum, which we refer to as “rest of brain”, scales linearly with
the number of neurons in the primate spinal cord. This linearity
warrants the use of numbers of neurons in the rest of brain, which
are available for all 41 species in our sample, as a proxy for numbers
of neurons in the spinal cord and also for the increase in numbers of
neurons that would be directly related to any variations in body size
(regardless of whether total volume, sensory surface area, muscular
mass or energetic requirement is the relevant parameter). We thus
use numbers of neurons in these structures as an internal reference
for the examination of how information processing might scale
faster in the cerebral cortex and in the cerebellum than required for
dealing strictly with bodily functions, without having body mass as
a confounding variable” [13].
“the cerebral white matter, which contains not only axons but
around 2 billion neurons and a large, but unknown, number of
glia in humans, seems to increase dis-proportionately compared
to gray matter as brain size scales across species. Not surprisingly,
the cerebral neo-cortex and cerebellum, which contain the largest
amounts of white matter, tend to make up greater proportions of
larger mammalian brains” [14].
h. Harvey J Karten [15]
“Progressive Telecephalization of Function
By the end of the nineteenth century, Herrick et al had
demonstrated that the brain-stem of all vertebrates shared a
profound level of similarity. the thalamus and telencephalon,
except for the olfactory bulbs, seemed to show few commonalities
between mammals and non-mammalian vertebrates. This led to
the prevailing view that the forebrain of most non-mammalian
vertebrates was related to olfactory inputs. The mammalian
forebrain, particularly the cortex of the telen-cephalon, was
increasingly thought to be novel and unique to mammals. There was
no structure in the non-mammalian forebrain that could readily be
compared with the mammalian cortex. The belief in the uniqueness
of the mammalian forebrain was particularly emphasized in the
writings of Sir HJackson (1911), and his co-worker, D.Ferrier
(1928), who suggested that over the course of evolution, functions
of the brainstem were transferred to the forebrain. This was
referred to as the progressive tele-encephalization of complex
functions. Examples of such functions included the ability to decode
auditory inputs generated by vocal communication, visual pattern
recognition, visual stereopsis, deciphering complex somato-sensory
inputs and most notably, so-called higher cognitive functions. The
level of analysis performed was judged to be that requiring the
participation of the neo-cortex in mammals.
But how could non-mammalia perform such operations in the
absence of modal-specific thalamic nuclei and cortical regions?
Structures within the forebrain, such as the specific sensory relay
nuclei of the thalamus and the ‘neo-cortex’ of the telencephalon,
were largely considered unique to mammalian brains. The
telencephalae of non-mammalia were considered to consist almost
exclusively of olfactory centres and basal ganglia. This directly
implied the lack of refined lemniscal visual, vestibular, gustatory,
auditory or somatosensory inputs to the tele-encephalon, and
certainly no prospect of ability to deal with discrete stimuli from
any of these sources. It also posed a paradox among birds, as many
species of birds with large tele-encephalae have only very limited,
or no olfactory capabilities, particularly when compared with many
non-avian reptiles and mammals. What might be the possible
function of the large avian tele-encephalon?
the notion of the uniqueness of mammals with a distinct
thalamus and neo-cortex was based on painfully sparse information.
The afferent connections to the thalamus in non-mammalian brains,
their projections upon the telencephalon and the various discrete
populations of the telencephalon were almost totally unexplored.
This led to the erroneous notions that the thalamic and cortical
populations of the mammalian brain were unique to mammals and
arose abruptly with the evolutionary origin of mammals “[15].
Nat Rev Neurosci. Author manuscript; available in PMC 2008
Aug 12.
i. Jarvis ED et al. [16]
“They noted that the main divisions of the human CNS-the
spinal cord, hindbrain, midbrain, thalamus, cerebellum and
cerebrum or tele-encephalon-were present in all vertebrates.
Edinger, however, noted that the internal organization of the
telencephala showed the most pronounced differences between
species. In mammals, the outer part of the telencephalon was found
to have prominently layered grey matter whereas the inner part
had nuclear grey matter. The inner part was located ventrally to the
lateral ventricle. The outer part was more elaborate and folded in
humans than in smaller- mammals. In non-mammals, the outer and
inner parts of the tele-encephala were mainly composed of nuclear
grey matter, most of which was located ventrally to the lateral
ventricle in reptiles and birds On the basis of these considerations,
Edinger proposed that tele-encephalic evolution occurred in
progressive stages of increasing complexity and size, culminating
with the human cerebrum. He suggested that the stages proceeded
in a ventral-to-dorsal direction, with each new vertebrate group
acquiring a more advanced cerebral sub-division, much as the
earth’s geological strata formed over time. He proposed that,
first, there was the old brain, the palaeo-encephalon (also called
the basal ganglia or subpallium at the telencephalic base), which
controlled instinctive behaviour, followed by the addition of a new brain, the neo-encephalon (also called the pallium or mantle at the
top of the tele-ecephalon), which controlled learned and intelligent
behaviour. He, A.Kappers and others named the tele-encephalic
subdivisions within each vertebrate group with the prefixes
‘palaeo’ (oldest),‘archi’ (archaic) and ‘neo’ (new) to designate
the presumed relative order of evolutionary appearance of each
subdivision. In Greek, ‘archi’ means the oldest, the first, or the
most primitive, whereas ‘palaeo’ meansancient, primitive or old,
but not necessarily the oldest. Both Edinger and AriënsKappers
misinterpreted the meaning of these prefixes and reversed them,
naming structures with ‘palaeo-’ to indicate the oldest or first and
‘archi-’ to indicate old. They added to these prefixes the root word
‘striatum’ for the presumed palaeo-encephalic subdivisions and
‘pallium’ or ‘cortex’ for the presumed neoencephalic subdivisions.
The term ‘striatum’ was used because a large part of the basal
ganglia (palaeo-encephalon) in mammals, now commonly called
the caudate–putamen, has fibre bundles coursing through it that
give it a striated appearance.
The classical view that became dominant was that the primordial
telencephalon of fishes had a relatively small pallium and a larger
sub-pallium, both of which were entirely devoted to olfactory
information processing. The fish sub-pallium was named ‘palaeostriatum’
(old striatum) and was thought to be the antecedent of
the human Globus pallidus. Amphibians were thought to have
evolved an ‘archi-striatum’ (archaic striatum) above the palaeostriatum,
which was proposed to be the antecedent of the human
amygdala. Reptiles were thought to have evolved a ‘neo-striatum’
(new striatum) above the archi-striatum, which was proposed to
be the antecedent of the human caudate and putamen. The palaeostriatum
of reptiles was also thought to have elaborated into an
older part (primitivum) and a newer part (augmentatum), both of
which were considered homologous to the human globuspallidus.
Following this, birds were thought to have evolved a large additional
basal ganglia subdivision, the ‘hyperstriatum’ (hypertrophied
striatum), which was considered to be unique to birds.
The fish pallium was named ‘palaeo-cortex’ and was proposed
to be the antecedent of the human olfactory cortex. Reptiles were
thought to have evolved an ‘archi-cortex’, also thought to be olfactory
and primitive, that was said to be the antecedent of the human
hippocampus. Birds were thought not to have evolved any further
pallial regions. By contrast, mammals were thought to have evolved
the latest and greatest achievement, a ‘neocortex’, from the palaeocortex
and/or archicortex6. The archi-cortex and/or palaeocortex,
with their 2–3 cell layers, were assumed to be primitive; the neocortex,
with its 6 layers, was assumed to be more recently evolved
and a substrate for more sophisticated behavior
There were dissenting voices to the classical view. Some of its
proponents also made partial or tentative retractions. alternative
views were not widely embraced. Instead, the classical view was
codified in the important 1936 comparative neuro-anatomy text by
A Kapperse et al and became pervasive throughout neuroscience. A
new view of telencephalic evolution.
With this new understanding of the avian telencephalic
organization and its homologies with that of mammals, we can
generate more informed hypotheses and conclusions about
telencephalic evolution in vertebrates. It is now apparent that
the organization of the true basal ganglia among birds, mammals
and other vertebrates (that is, distinct nuclear striatal and pallidal
domains with more dopaminergic input into the striatal domain)
is quite conserved. By contrast, the organization of the pallial
domains of these groups is more varied. The avian hyperpallium
has a unique organization that has so far been found only in birds.
This consists of semi-layered subdivisions and might have evolved
more recently than the mammalian six-layered cortex, as birds
evolved ~50–100 million years after mammals. The DVR (which, in
birds, contains the meso-pallium, nido-pallium and arco-pallium)
is a nuclear, grey matter formation that is unique to birds and
reptiles. The six-layered cortex is unique to mammals, and, as all
the main groups of living mammals (monotremes, marsupials
and placentals) have a six-layered cortex87, it was presumably
inherited from their common therapsid ancestor more than
200 million years ago. Furthermore, new findings indicate that
mammals did not arise from reptiles, but from therapsids, and
that the last common ancestor of the reptile and mammal lineages
was the stem amniotes. As all non-mammalian therapsids are now
extinct, it is difficult to trace from stem amniotes to mammals the
evolutionary history of mammalian tele-encephalic organizationlayered,
nuclear or otherwise. Therefore, the reptilian nuclear
pallial organization cannot be assumed to represent the ancestral
condition for mammals “[16].
j. Fahima Mayer et al. [17]
Pharmacologic remedy of many brain diseases is difficult
because of the powerful drug exclusion properties of the blood–
brain barrier (BBB). Chemical isolation of the vertebrate brain is
achieved through the highly integrated, anatomically compact and
functionally overlapping chemical isolation processes of the BBB.
These include functions that need to be coordinated between
tight diffusion junctions and uni-directionally acting xenobiotic
transporters. Understanding of many of these processes has been
hampered, because they are not well mimicked by ex vivo models
of the BBB and have been experimentally difficult and expensive
to disentangle in intact rodent models. Here we show that the
Drosophila melanogaster (Dm) humoral/CNS barrier conserves
the xenobiotic exclusion properties found in the vertebrate
vascular endothelium. We characterize a fly ATP binding cassette
(ABC) transporter, Mdr65, that functions similarly to mammalian
xenobiotic BBB transporters and show that varying its levels solely
in the Dm BBB changes the inherent sensitivity of the barrier
to cytotoxic pharmaceuticals. Furthermore, we demonstrate
orthologous function between Mdr65 and vertebrate ABC transporters by rescuing chemical protection of the Dm brain with
human MDR1/Pgp. These data indicate that the ancient origins of
CNS chemoprotection extend to both conserved molecular means
and functionally analogous anatomic spaces that together promote
CNS selective drug partition. Thus, Dm presents an experimentally
tractable system for analyzing physiological properties of the BBB
in an intact organism.
In vertebrates, a physically separate blood–brain barrier
(BBB), primarily engineered into the single-cell layer vascular
endo-thelium (VE), provides anobstacle to chemical attack. At this
interface, strong selective pressures have produced the integration
of at least two very different cell biologic mechanisms to prevent
free movement of small molecules between the humoral and CNS
interstitial compartments. BBB VE cells impede the traffic of drugs
by virtue of specialized lateral junction components, including
tight junctions, and asymmetrically arrayed ATP binding cassette
(ABC) transporters. Tight junctions prevent para-cellular diffusion
of charged molecules, and asymmetrically arrayed transporters
actively expel lipophilic molecules backinto the humoral space.
Together, these complimentary systems prevent the majority of
xeno-biotics from acting on vertebrate nervous tissue. Although
in vivo and in vitro BBB models have confirmed the importance of
these two components, substantial limitations hinder progress. A
powerful BBB model system should combine molecular genetics,
genomic, chemical biology, and integrative physiology tools to
probe CNS-specific chemo-protective physiology. For this, we
turned to Drosophila melanogaster (Dm) and asked what aspects
of BBB physiology can be modeled in an in-vertebrate.
Insects also possess protective neural barriers, but they differ
anatomically from vertebrates. Dm has an open circulatory system
that is separated from the CNS by a thin layer of glially derived
epithelial cells making the Dmhumoral/CNS interface topologically
much simpler than the vertebrate BBB. on a cellular level, the
vertebrate and insect BBBs share many common features. one
specific cell layer of the Dm BBB, the subperineural glia (SPG),
possesses elaborate laterally localized homotypic junctional
complexes, or pleated septate junctions, that create a tight barrier
to para-cellular diffusion The Dm proteins that make up the pleated
septate junctions are nearly identical to the vertebrate proteins
that compose the tight junctions .disruption of the pleated septate
junctions leads to defects in Dm BBB function but the dual nature
of localized xenobiotic protection mechanisms had not been
established in insects” [17].
k. Bufill E et al. [18]
“Alzheimer’s disease is a complex disease associated with
advanced age whose causes are still not fully known. Approaching
the disease from an evolutionary standpoint may help in
understanding the root cause of human vulnerability to the disease.
AD is very common in humans and extremely un-common in other
mammals, which suggests that the genetic changes under-lying the
alterations in cerebral structure or function that have taken place
over the course of the evolution of the genus Homo have left specific
neurons in the human brain particularly vulnerable to factors
which trigger the disease. Most of the genes whose mutation leads
to AD are involved in synaptic plasticity. Evidence has also been
found relating AD to neuronal oxidative stress. Neurons in certain
association areas of the human brain retain juvenile characteristics
into adulthood, such as the increased expression of genes related
to synaptic activity and plasticity, incomplete myelination and
elevated aerobic metabolism, which can cause an increase in
oxidative stress in these neurons. Oxidative stress can cause
myelin breakdown and epigenetic changes in the promoter region
of genes related to synaptic plasticity, reducing their expression.
These changes may in some cases induce hyper-phosphorylation
of tau and β-amyloid deposits, which are characteristic of AD. The
adaptation of humans to the cognitive niche probably required an
increase in synaptic plasticity and activity and neuronal metabolism
in neurons in areas related to certain cognitive functions such as
autobiographical memory, social interaction and planning. The cost
of these changes may have been the brain’s increased vulnerability
to factors which can trigger AD. This vulnerability may have
resulted from the evolutionary legacies that have occurred over the
course of the evolution of the human brain, making AD a possible
example of antagonistic pleio-tropy. The evolutionary approach
allows apparently unrelated data from different disciplines to be
combined in a manner that may lead to an improved understanding
of complex diseases such as Alzheimer’s” [18].
l. Mark R Cookson [19]
“There are a number of neuro-degenerative diseases that
principally affect humans as they age, characterized by the loss
of specific groups of neurons in different brain regions. Although
these are in general sporadic disorders, it is now clear that many
of these diseases have a substantial genetic component. As genes
are the raw material with which evolution works, we might benefit
from understanding these genes in an evolutionary framework.
Here, I will discuss how we can understand whether evolution has
shaped genes involved in neurodegeneration and the implications
for practical issues such as our choice of model systems for these
diseases and more theoretical concerns such as the level of selection
against these phenotypes.
Evolutionary theory, modified to include our modern
molecular views on genetics, permeates all aspects of modern
biology. Understanding human biology therefore incorporates
acknowledgement of our genetic heritage shaped by the
evolutionary forces that have led to humans occupying our current
niche. And it should not be surprising that, as a major aspect of our
biology, the near universal experience of human disease can also be
viewed through the evolutionist’s prism.
I will discuss the relationship of evolution to age-related neurodegenerative
disorders. This group of diseases is characterized by the shared property in the progressive loss of relatively specific
groups of neurons. What distinguishes each is that during the aging
process different groups of neurons are lost in each disease and
these correlate with different clinical features. neuron loss in the
hippocampus and cerebral cortex underlies many of the memory
problems associated with AD whereas ataxia is a consequence of
loss of Purkinje cells in the cerebellum and is characteristic of the
spino-cerebellar ataxias. These symptoms are often profoundly
disabling and sometimes fatal; loss of the neurons that innervate
the diaphragm in amyotrophic lateral sclerosis (ALS) leads to an
inability to breathe.
in many but not all neurodegenerative conditions, there are
other pathological events including the accumulation of specific
proteins in those neurons that survive. Often these are aggregated
and insoluble and, more importantly, often the genes that code for
these pathological proteins either cause inherited forms of disease
and/or act as genetic risk factors. pathology, clinical phenotype and
causal variation in specific genes are linked.
There are two questions to discuss here to understand
the genes and proteins associated with neurodegeneration in
the context of evolution. The first is in what ways can we use
evolutionary views on sequences to understand aspects of protein
function and dysfunction related to mutations associated with
neurodegenerative diseases. This has practical implications, for
example in assigning pathogenicity to specific mutations in genes
or for understanding how far we can extrapolate from model
systems to human diseases. The second, consideration is whether
evolutionary forces have shaped these degenerative diseases. To
provide a detailed example, I will first cover Parkinson’s disease,
a disorder that illustrates many of the key points under discussion
here. I admit this is biased by my own research interests, so refer
the interested reader to other review articles about the genetics of
neuro-degenerative diseases more generally” [19].
DETOXING FOR BRAIN HEALTH – NEW RESEARCH FINDINGS:
Craniosacral Therapy Improves Glymphatic Cleansing of Brain
Tissue
m. Carolyn Simon
“New research provides evidence the body has a fast-track brain
cleansing system that prevents diseases such as Alzheimer’s and
maintains brain health. Finding ways to support and enhance this
cleansing process could lead to improved outcomes in brain injury
and brain disease. Read on to find what scientists have discovered
and how cranio-sacral therapy effectively promotes brain health by
invigorating this active fluid -cleansing system.
Most of us have heard of the lymphatic system, the collection of
vessels and nodes running throughout the body that helps cleanse
waste products and is part of the body’s immune system. Now a
team of neuroscientists at the Univ. of Rochester Medical Center
has identified a fascinating fast-track cleansing system in the brain
called the glymphatic system. A bulk flow process moves CSF via
the arterial system right into the brain tissue, exchanging with the
interstitial fluid inside the brain. As it does, it washes through the
tissue collecting waste particles that are sitting in between the
brain cells. The CSF then enters the venous system via veins within
the brain tissue, taking the fluid and the waste it picks up away from
the brain. In this way waste material is efficiently removed from
the brain tissue, by the CSF, via the circulatory system. We know
accumulation of waste and toxic matter in the brain environment
adversely affects brain- function”.
Form an Article published in Scientific American https://www.
scientificamerican.com/article/brain-cleaning-discovery/
Brain’s Drain: Neuroscientists Discover Cranial Cleansing
System
“Fluids coursing through the nervous system could help clear
the brain of toxic detritus that leads to Alzheimer’s and Huntington’s
disorders 2012.
The brain can be a messy place. Thankfully, it has good
plumbing: Scientists have just discovered a cleansing river inside
the brain, a fluid stream that might be enlisted to flush away the
buildup of proteins associated with Alzheimer’s, Huntington’s and
other neuro-degenerative disorders
The researchers, based at the University of Rochester (U.R.),
University of Oslo and Stony Brook University, describe this
new system in the journal Science Translational Medicine today.
The study adds to the evidence that the star-shaped cells called
astrocytes play a leading role in keeping the nervous system in
good working order.
In most of the body, a network of vessels carries lymph, a fluid
that removes excess plasma, dead blood cells, debris and other
waste. But the brain is different. Instead of lymph, the brain is
bathed in cerebro-spinal fluid.
n. Walker S Jackson [20]
“The mechanisms under-lying the selective targeting of specific
brain regions by different neuro-degenerative diseases is one of the
most intriguing mysteries in medicine. it is known that A D primarily
affects parts of the brain that play a role in memory, whereas PD
predominantly affects parts of the brain that are involved in body
movement. The reasons that other brain regions remain un-affected
in these diseases are unknown [20] (Figure 5-8
).
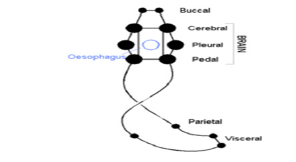
Figure 5:Nervous system in gasteropodes (torsion).
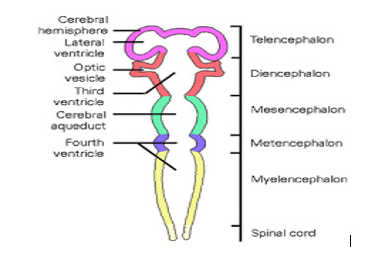
Figure 6:CNS embryology
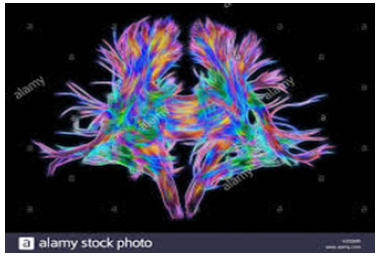
Figure 7:White matter fibres-computer-enhanced-3d-diffusionspectral-
imaging
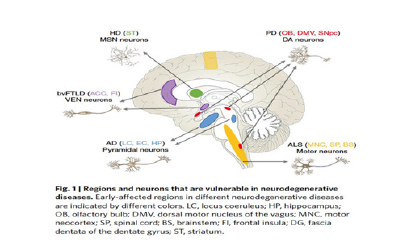
Figure 8:Neuronal vulnerability
Experimental project hypothesis:
To verify the hypothesis that evolution pattern is involved in
some neuronal vulnerability is possible to observe the incidence of
cortical neuro degenerative pathology across the various superior
vertebrates ( in example related the increase of cortical cognitive
advanced function) then compare this data also with the incidence
of other degenerative process in inferior vertebrates.

 Creative Commons, CC-BY
Creative Commons, CC-BY











 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.