Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
New Promising Horizons for the Antitumor Activity of Onconase
*Corresponding author: Giovanni Gotte, Department of Neuroscience, Biomedicine and Movement Sciences, Biological Chemistry Section, University of Verona, Strada Le Grazie, 8, 37134 Verona, Italy.
Received: April 01, 2020; Published: April 28, 2020
DOI: 10.34297/AJBSR.2020.08.001308
Abstract
Cancers represent, together with cardiovascular diseases, the most important cause of death in the industrialized countries. Fortunately, many cancer types have been successfully counteracted by following different strategies, comprising chemio- and/or radio-therapies, new drugs design and immuno-therapy. The success of these therapies, however, often depends on early diagnosis, that is very difficult to get for some tumors, especially the ones affecting internal organs, such as lung, ovary, liver, pancreas. Therefore, the curability of these tumors remains low and, consequently, the related deaths high. In this scenario, although rarely representing the first choice, protein therapy could be a fruitful approach to counteract incurable tumors. RNases, which are able to attack many RNA types, can become tools to block an uncontrolled cell replication and, consequently, cancer development. In particular, the amphibian RNase ranpirnase, commonly called onconase (ONC), showed in the recent past to be active against many tumors either in vitro or in vivo. Nevertheless, its renal toxicity, although reversible, has limited its use in therapy. However, the most recent results obtained in vitro with ONC are presented here, and possible therapeutic strategies based on ONC self- or hetero-oligomerization are as well suggested to overcome renal toxicity.
Keywords: Ribonucleases; Onconase; Antitumor Activity; ImmunoRNases; Oligomeric RNases; 3D Domain Swapping
Abbreviations: AA: Aminoacid; RNase(s): Ribonuclease(s); ONC: Onconase; ANG: Angiogenin; pt-RNases: Pancreatic type-RNases; BS-RNase: Bovine seminal RNase; RI: RNase Inhibitor; EDC: 1-ethyl-3-(3-dimethylaminoisopropyl) carbodiimide
Introduction
Ribonucleases
Ribonucleases (RNases) form a very large bacterial or eukaryotic enzymes group [1] and are known to catalyze the hydrolysis of many RNA substrates [2]. This makes their classification not easy, also because a cell contains about twenty different distinct RNases often characterized by different substrate specificities [3]. However, a possible classification can differentiate intracellular RNases from the ones secreted extracellularly. These are called secretory RNases [1,4] and many of them form a large super-family [5] in which is also included an amphibian RNase, called Onconase, that displays a remarkable antitumor activity [6,7]. Its main features are described in this report.
Pancreatic-type RNases and Onconase: crucial features for cytotoxicity
In the group of the secretory RNases, an increasing number have been characterized since the 60ies, and many of them have been classified as “pancreatic-type” (pt)-RNases [5,8]. This term originates from the most studied enzyme, the 13.7 kDa and 124 aminoacid (AA) residues-long bovine pancreatic, monomeric RNase A (Figure 1A) [9,10]. Incidentally, the members of this super-family refer sometimes to human pancreatic RNase, called HP-RNase, or RNase 1 (Figure 1B) [8,11]: although displaying a high identity sequence with RNase A, it is definitely more basic than it and is not expressed only in the pancreas, but almost in all tissues [12]. Besides RNase A and RNase 1, other variants are included in the mentioned super-family, even if, again, some of them are not secreted by the pancreas. The most important members display remarkable biological activities, as it is for the natively dimeric cytotoxic bovine seminal RNase (BS-RNase), that exists as an equilibrium between two isoforms, as reported in (Figure 1C) [13,14]. and for human RNase 5 [15]. This latter variant is also called angiogenin (ANG, (Figure 1D) because it crucially contributes to the formation of new blood vessels thanks to its ribonucleolytic activity [16,17]. Also other important RNases, although belonging to non-mammalian species, such as birds or amphibians, are known [18,19]. Some of them are included in the pancreatic-type super-family principally because of their high structural homology with the mammalian pt- RNases [6]. In particular, the 114 AA residues amphinase and, above all, the 104 AA residues frog ranpirnase, or P-30 protein, extracted from Rana Pipiens oocytes, deserve to be noted [7,20]. This 11.8 kDA variant is commonly called onconase (ONC, (Figure 1F) because it exerts a remarkable antitumor activity against many cancer types [6,7]. Moreover, ONC is known to display also an antiviral activity against HIV-1 or, more recently, Ebola [21,22]. ONC is considered a pt-RNase because it satisfies the three main features for which a RNase can be associated with the super-family [5]:
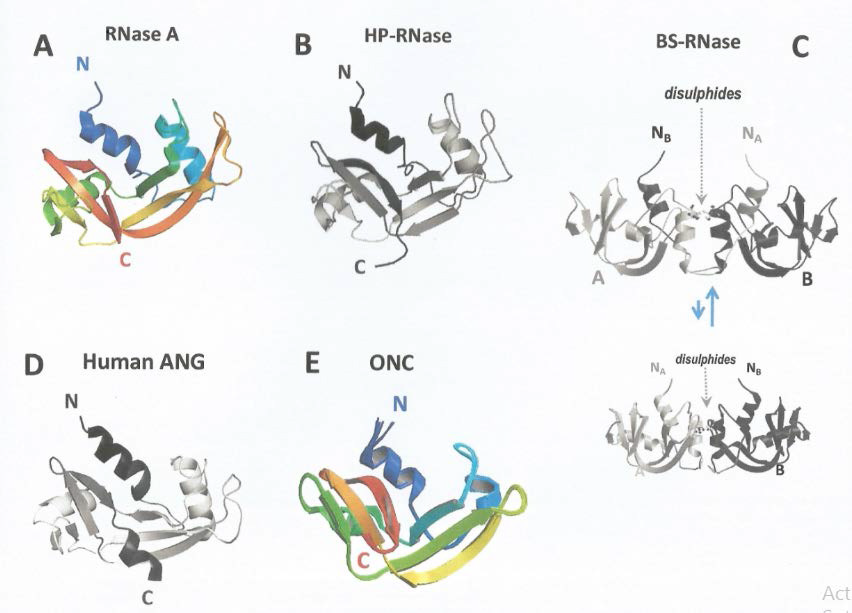
Figure 1: Structures of some important pt-RNases.
RNase A; B) HP-RNase, or RNase 1; C) natively dimeric BS-RNase: two isoforms in equilibrium exist, one natively swapping their N-termini, the
other dimeric only through the indicated disulphides; D) human Angiogenin, ANG; E) amphibian Onconase, ONC.
1. a high homology folding represented by a “V-like”, or
“kidney-like”, shape to accommodate the RNA substrate in the
relative cavity [23],
2. the catalytic triad, formed by one Lys and two His residues,
i.e., H10/K31/H97 for ONC, while for RNase A is H12/K41/
H119,
3. the distribution of the basic charged residues, the majority
of which must be located in the proximity of the active site
[5]. Importantly, at least a minimal ribonucleolytic activity is
mandatory for RNases to exert their remarkable biological
activities, as it is for ANG [24], but also for ONC [6]. Indeed,
ONC cytotoxicity against malignant cells definitely emerges
[25-27]. In this context, beyond BS-RNase and, especially, ONC,
also the bacterial Barnase and the natively dimeric Binase
[28], are definitely cytotoxic [29]. However, ONC is the most
considered RNase anticancer tool [30].
Determinants of the Antitumor activity of pt-RNases and ONC
The efficacious action of ONC revealed to be particularly true for incurable solid tumors, as emerged from the use of ONC against human lymphoma [31], glioma [32], pancreatic carcinoma [33], or, more recently, melanoma cell lines [34,35]. In particular, an autophagic cell death effect has been detected in ONC-treated Panc-1 and PaCa-44 tumor cells [33], while ONC affects also NF- κB and TNF-α expression in A375 melanoma cells [34,35]. Strong synergism was reported in early studies with ONC combined in vitro with tamoxifen [36], or trifluoroperazine [37], or also lovastatin [38] to counteract pulmonary A549 carcinoma cells. Importantly, an ONC antitumor action had been registered also in vivo, in particular against non-resectable mesothelioma and nonsmall- cell lung cancer [39-41]. However, this application resulted to be not completely successful because nephrotoxicity emerged in many patients after repeated ONC administrations, although this side-effect disappeared after discontinuing the treatment [42].
Cellular internalization
The main obstacle encountered by extracellular RNases to exert their action is represented by cell internalization. This occurs through endocytosis [43], but is possible only through a fruitful interaction with the cell membrane occurs. However, the possibility for ONC to enter the cell thanks to the mediation of a receptor has been reported as well [44,45]. Then, Sundlass et al. [46] revealed that both electrostatic forces and specific interactions are crucial for a RNase to determine the time spent near the cell surface, a determinant for its consequent internalization [46]. In addition, Notomista & coll. reported that either native or artificial dimeric cytotoxic RNases strongly affect membrane aggregation, fluidity and fusion [47]. Importantly, if we consider that a RNase should be selectively cytotoxic against malignant cells, that are characterized by a more negatively charged membrane than the normal ones, the basicity of each RNase is important to win this challenge. Then, also the specific RNase orientation is important for a successful approach to the membrane, as it has been demonstrated for ONC or also BS-RNase [46-49]. Hence, the orientation of the basic charges might affect also the cytotoxic potential of other RNases. In addition, ONC seems to approach the cell membrane differently from other pt-RNases [46]. Moreover, the cellular internalization event can be evaluated as to be residue-specific because wt-ONC is less efficiently internalized than the so called “R-mutant”, in which all Lys residues except the catalytically active one are replaced by arginines [50]. However, if the RNase net basic charge is randomly increased, the relative advantage can be counteracted by the increase of the enzyme affinity toward the negatively charged cellular RI [51].
Some discordant data have been reported about the mechanism of ONC cell internalization: Haigis & Raines wrote that ONC is internalized in early endosomes of HeLa and K562 cells by a clathrin- and caveolae-independent mechanism [45], while Rodriguez & coll. reported that Jurkat cells can endocytate ONC through a dynamin-dependent route, presumably through a pathway mediated by clathrin/AP-2 [52]. These data, although apparently controversial, suggest that ONC may follow different routes to cross the membrane of different cell lines.
Evasion from the RNase inhibitor
Another huge obstacle for the biological activity of a RNase is represented by its interaction with the cellular RNase Inhibitor (RI). RI is a 50 kDa negatively charged, horseshoe-shaped, and cysteineplus leucine-rich macromolecule ubiquitously expressed in almost all cells [53,54]. For many years, RI has been considered present only in the cytosol, but its presence has been detected also in cell mitochondria and nuclei [55]. RI can form very tight complexes with many pt-RNases, such as RNase A [56], RNase 1 [57], ANG [58], and also with RNase 2, that is the eosinophil derived neurotoxin (EDN) [59]. The RNase-RI complexes are accompanied with Kd values comprised between the pico- and the femto-molar range [60]. Their structures explain why RI inactivates the RNase moiety that remains caged inside the RI cavity [61]. RI is highly conserved in mammals, but is present also in non-mammalian species [62]. Instead, in contrast with almost all secretory pt-RNases, ONC can evade RI because it is devoid of the flexible regions, or loops, in which reside the key-residues allowing RNases to fruitfully interact with RI [44,61]. For this reason, ONC can actually display a remarkable cytotoxicity by exerting its ribonucleolytic activity toward t-RNAs [63] and arresting the G1 cell cycle phase [7,20]. Besides, it is important to note that also the mammalian BS-RNase is cytotoxic because, being natively dimeric, can sterically evade RI [64,65]. On the contrary, its monomeric derivative, although being enzymatically active, is not cytotoxic because is sequestered by RI [66,67]. However, the sensitivity toward RI is not the unique determinant hindering cell cytotoxicity: indeed, non-cytotoxic RNases were unable to reduce HeLa cells viability also after silencing the RI expression [68]; instead, non-covalent dimers of RNase A, although being partially RI-sensitive [69] and definitely inactive against pancreatic cancer cell lines [70], showed to be cytotoxic against leukemia cells and also against melanoma in mice [71,72].
Native or artificial oligomeric RNases and their possible application in therapy
If a RNase can be induced to oligomerize, this would make it bulkier than its native monomer, and the charge density of its moiety augment likewise. This event would help the enzyme internalization in tumor cells and would allow its evasion from the cellular RI as well [67]. Then, the augment of ONC derivatives dimensions could represent a successful strategy if we consider that ONC-based therapy had been limited by adverse effects at the expense of the kidneys: ONC antitumor activity would be conjugated, in fact, with a simultaneous low renal uptake. Protein oligomerization may occur spontaneously, or could be induced also by the cell environment or, again, by an in vivo context [65,73,74]. Within mammalian RNases, only BS-RNase is nowadays known to be natively homo-dimeric [14]. This is principally ascribable to two antiparallel disulphides involving the two Cys31 and 32 residues that are present in both subunits [13,75], while are absent in ONC and also in other monomeric RNases.
Covalent or non-covalent ONC oligomerization
RNase oligomerization can be provoked also in vitro to obtain different products. Incidentally, RNases or ONC oligomerization can be induced to form covalently linked derivatives upon conjugation with bifunctional or multifunctional cross-linkers, like diimidoesters or maleimides. In this way, stable hetero- or homo-oligomers can be produced, upon modifying one or more AA residue(s). Therefore, any chemical modification can somehow affect the properties of native RNases and, thus, negatively affect its biological activities. Nevertheless, this approach has been often exploited by RNase A permitting to obtain products displaying promising, but not conclusive, results in terms of high cytotoxic potential. This was, very probably, because of the excessive involvement and modification of the basic Lys residues [76-78]. Again, maleimides have been instead used with a properly designed ONC mutant to obtain bulky dimer(s) or trimer(s) (Figure 2A) that, however, were not more cytotoxic, in vitro, than monomeric ONC [79]. Nevertheless, and importantly, these derivatives were characterized also by dimensions that overpassed the calibre of the glomeruli [79], and their use could be reconsidered in the next future.
Immuno-RNase protein fusion strategy for ONC
To obtain larger RNase moieties without affecting their activity properties, the protein-fusion technique has been widely used especially with RNase 1 [80,81], but sometimes also with ONC [82]. Thanks to protein engineering, ONC derivatives have been designed with different peptide linkers and expressed in conjugation with many adducts [82], such as antibody fragments, human serum albumin, dengue virus-derived peptide, and also the transferrin N-terminal domain ((Figure 2B), upper panel) [83-85]. All derivatives displayed remarkable cytotoxicity against many cancer cell types, and in some cases also in an in vivo context, in mice [83,86]. Furthermore, this augmented antitumor activity could have been paralleled, in a possible in vivo application, by a low undesired renal filtration [79]. Then, differently from microbial or plant immunotoxins, human Immuno-RNases lack immunogenicity or nonspecific binding and toxicity that could damage also normal cells [87]. Indeed, clinical trials performed with non-mammalian toxins drove sometimes toward even fatal events [88]. Instead, the Immuno-RNase 1 fusion derivatives were benign outside cells, and not immunogenic as well [80]. Furthermore, the immuno-protein fusion approach has been applied also to form ONC “diabodies” (Figure 2B), lower panel), i.e., covalent dimers of single chain antibody fragments (scFv) connected with the RNase moiety, or in other words dimers of the Immuno-ONC derivatives [86,89].
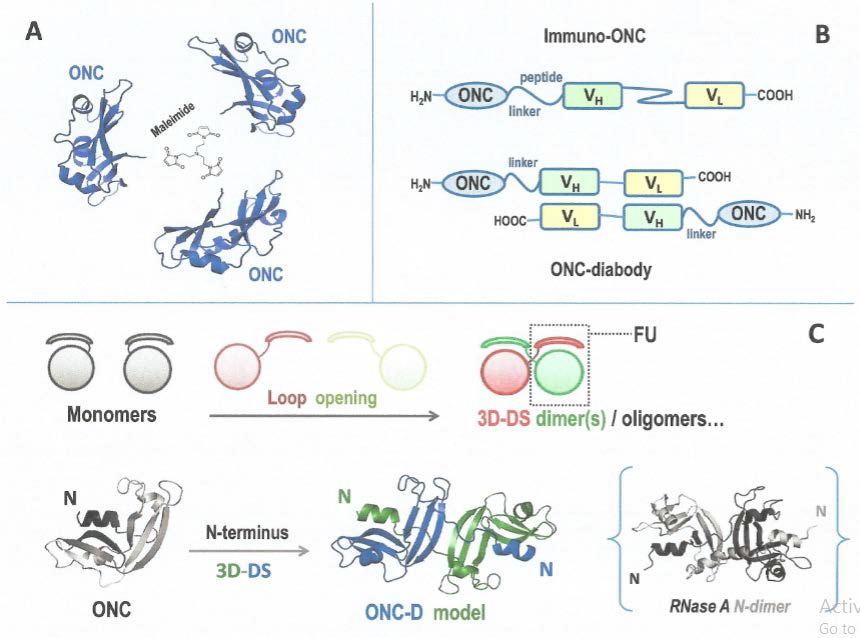
Figure 2: Strategy for ONC oligomerization.
A) Covalent oligomerization (trimerization) of ONC; adapted from [79]; B) Immuno - RNase (ONC) fusion protein [84,85] and ONC diabody schematic
structures [86,89]; C) 3D-DS mechanism [91,95-97] and N-swapped ONC dimer model [105] compared with the crystal structure of the RNase A
N-dimer [107]. FU represents the functional unit reconstituted in the dimer after 3D-DS [96].
Oligomerization through the three dimensional domainswapping (3D-DS) mechanism
RNases can form supramolecular structures also through a non-covalent self-association mechanism called three dimensional domain-swapping (3D-DS). Firstly described and analyzed by Eisenberg, but then also by other groups [90-95], 3D-DS involves many proteins [90,96,97] and partially violates the “Anfinsen dogma” which states that a protein AA sequence dictates a unique folding [98]. Indeed, the flexible loop(s) of a protein can adopt variable conformations corresponding to more than one energy minimum [91]. This possibility allows the domains linked to the flexible protein segments to adopt different orientations and undergo a reciprocal exchange (Figure 2C), upper panel). This drives to form a non-covalent dimer, or dimers, or even larger oligomers, as it is for RNase A and BS-RNase [65,99-101] if more than a single flexible loop is present [96,97]. The domain detached from the native monomer can reconstitute the original contacts in each composite, functional unit (FU) of the oligomer [96]. The FU overlaps the native monomer (Figure 2C), upper panel), while the folding of the dimer/oligomer parallels the monomer one, with the exception of additional, so called open interface(s) [91]. The domains involved in 3D-DS are often the protein N- or C-termini, or both, as it is, again for RNase A and BS-RNase [65,96,99,101]. Oligomerization is often accompanied with increased RNases enzymatic and biological activities, being the latter ones sometimes absent in the native monomer [72].
3D-DS dimerization of ONC: ONC can penetrate cancer cells either for its high basicity or to a favorable interaction with the sialic acid moieties present on the membrane of malignant cells [102]. Afterwards, ONC evades RI as mentioned before [60] and can attack tRNAs or other substrates, such as miRNAs, to exert its cytotoxic action [21,103,104]. Hence, it would seem not necessary to produce ONC oligomers to design anticancer therapies [30]: indeed, many positive results have been reached with monomeric ONC both in vitro and in vivo against several incurable tumors [82]. Furthermore, ONC was recently found to enhance the activity of new generation drugs that are active against the BRAF-mutated A375 melanoma cell line [34], and also to restore cytotoxicity vs the same A375 cells that became resistant to dabrafenib [35]. However, the although reversible renal toxicity lowered ONC therapeutic applications [42], and the possibility to enlarge the dimensions of ONC moiety/ies remains a promising strategy to allow a more efficacious block at the glomerular barrier. This would increase the half-life of circulating ONC derivatives at the same time, and the aforementioned fusion immune-ONC derivatives are in line with this strategy [83]. Then, notwithstanding its remarkable stability (TM ~90°C), ONC has been discovered to form a N-swapped dimer (ONC-D, (Figure 2C), lower panel) [105], upon being lyophilized from 40% acetic acid solutions, like RNase A [106]. The ONC-D structure has been modeled [105] as to be similar to the N-swapped dimer of RNase A (Figure 2C), lower panel) [107]. Notably, low concentrations of ONC-D displayed to be more active against pancreatic cancer cells than native monomer [105]. Unfortunately, ONC can swap only its N-terminus, because its C-terminus is locked by a disulphide bond involving Cys87 and Cys104, i.e., the last AA residue. The impossibility to swap more than one domain definitely reduces the self-association propensity of a protein [97]. Moreover, some ONC variants lacking the disulphide blocking the C-terminus are known to be less stable than the native enzyme [108,109]. Consequently, the only way nowadays feasible to obtain large ONC homo-oligomers is the use of multifunctional maleimides producing covalent, stable derivatives (Figure 2A) [79]. However, a recent study has combined the features of the ONC C-terminal loop with the ones of RNase A to build a chimera that increased the tendency of the mammalian enzyme to oligomerize through 3D-DS. Indeed, the RNase A native cis configuration of the Pro114 residue residing inside the flexible 112-115 residues loop makes its C-terminus difficult to be swapped [110], therefore requiring high energy to succeed [111,112]. Instead, the loop present in ONC, shorter and devoid of this proline residue, makes the RNase A mutant more prone to oligomerize [113]. The antitumor activity of the resulting oligomers was not tested because mutations did not affect the determinants crucial for cytotoxicity. Nevertheless, this result may suggest to deepen the analysis and combine the most advantageous features of RNase A and ONC. This could allow to build chimeras by combining the cytotoxic properties of ONC with the determinants making pt-RNases prone to oligomerize, and contemporarily reduce undesired side-effects. Once oligomers larger than dimer(s) being obtained, these species could be covalently stabilized by using condensing agents, as it had been performed with the RNase A C-dimer [114] with 1-ethyl 3-(3-dimethylaminoisopropyl) (EDC) carbodiimide [115].
Conclusion
The new data registered in the last decade by measuring the
in vitro ability of ONC to counteract incurable tumors are certainly
promising [30-32,34,35,70]. However, to obtain more satisfactory
results, the following strategies could be experimented:
I. artificial protein cross-linking (Figure 2 A)
II. immuno-fusion protein derivatives (Figure 2B),
III. protein engineering devoted to enlarge the tendency
of ONC 3D-DS self-association (Figure 2C). This could allow
to design and produce active derivatives that may become
efficacious tools able to counteract incurable cancers , but with
negligible side effects.
Acknowledgement
This work has been supported by the Italian Ministry of University and Research (MIUR), with the “Fondo Unico per la Ricerca” FUR-GOTTE.
Conflict of Interest
The author declares no conflict of interest.
References
- D Alessio GRJF (1997) Ribonucleases: Structures and Functions. Academic Press, New York, USA.
- Barnard EA (1969) Biological function of pancreatic ribonuclease. Nature 221: 340-344.
- Deutscher MP, Li Z (2001) Exoribonucleases and their multiple roles in RNA metabolism. Progress in nucleic acid research and molecular biology 66: 67-105.
- Beintema JJ, Schuller C, Irie M, Carsana A (1988) Molecular evolution of the ribonuclease superfamily. Progress in biophysics and molecular biology 51(3): 165-192.
- Sorrentino S, Libonati M (1994) Human pancreatic-type and Nonpancreatic-type ribonucleases: a direct side-by-side comparison of their catalytic properties. Archives of biochemistry and biophysics 312(2): 340-348.
- Ardelt W, Mikulski SM, Shogen K (1991) Amino acid sequence of an anti-tumor protein from Rana pipiens oocytes and early embryos. Homology to pancreatic ribonucleases. The Journal of biological chemistry 266(1): 245-251.
- Ardelt W, Shogen K, Darzynkiewicz Z (2008) Onconase and amphinase, the antitumor ribonucleases from Rana pipiens oocytes. Curr Pharm Biotechnol 9(3): 215-225.
- Beintema JJ, Kleineidam RG (1998) The ribonuclease A superfamily: general discussion. Cellular and molecular life sciences CMLS 54: 825-832.
- Smyth DG, Stein WH, Moore S (1963) The sequence of amino acid residues in bovine pancreatic ribonuclease: revisions and confirmations. The Journal of biological chemistry 238(1): 227-234.
- Raines RT (1998) Ribonuclease A. Chem Rev 98: 1045-1066.
- Beintema JJ, Wietzes P, Weickmann JL, Glitz DG (1984) The amino acid sequence of human pancreatic ribonuclease. Analytical biochemistry 136(1): 48-64.
- Morita T, Niwata Y, Ohgi K, Ogawa M, Irie M (1986) Distribution of two urinary ribonuclease-like enzymes in human organs and body fluids. Journal of biochemistry 99(1): 17-25.
- Suzuki H, Parente A, Farina B, Greco L, La Montagna R, et al. (1987) Complete aminoacid sequence of bovine seminal ribonuclease, a dimeric protein from seminal plasma. Biol Chem Hoppe Seyler 368(10): 1305-1312.
- Mazzarella L, Capasso S, Demasi D, Di Lorenzo G, Mattia CA, et al. (1993) Bovine seminal ribonuclease: structure at 1.9 A resolution. Acta Crystallogr D Biol Crystallogr 49(Pt 4): 389-402.
- Sorrentino S (2010) The eight human "canonical" ribonucleases: molecular diversity, catalytic properties, and special biological actions of the enzyme proteins. FEBS letters 584(11): 2194-2200.
- Fett JW, Strydom DJ, Lobb RR, Alderman EM, Bethune JL, et al. (1985) Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry 24(20): 5480-5486.
- Shapiro R, Riordan JF, Vallee BL (1986) Characteristic ribonucleolytic activity of human angiogenin. Biochemistry 25(12): 3527-3532.
- Cho S, Beintema JJ, Zhang J (2005) The ribonuclease A superfamily of mammals and birds: identifying new members and tracing evolutionary histories. Genomics 85(2): 208-220.
- Arnold U (2014) Stability and folding of amphibian ribonuclease A superfamily members in comparison with mammalian homologues. The FEBS journal 281(16): 3559-3575.
- Darzynkiewicz Z, Carter SP, Mikulski SM, Ardelt WJ, Shogen K (1988) Cytostatic and cytotoxic effects of Pannon (P-30 Protein), a novel anticancer agent. Cell Tissue Kinet 21(3): 169-182.
- Saxena SK, Gravell M, Wu YN, Mikulski SM, Shogen K, et al. (1996) Inhibition of HIV-1 production and selective degradation of viral RNA by an amphibian ribonuclease. The Journal of biological chemistry 271(34): 20783-20788.
- Hodge T, Draper K, Brasel T, Freiberg A, Squiquera L, et al. (2016) Antiviral effect of ranpirnase against Ebola virus. Antiviral Res 132: 210-218.
- McPherson A, Brayer G, Cascio D, Williams R (1986) The mechanism of binding of a polynucleotide chain to pancreatic ribonuclease. Science 232(4751): 765-768.
- Shapiro R, Vallee BL (1989) Site-directed mutagenesis of histidine-13 and histidine-114 of human angiogenin. Alanine derivatives inhibit angiogenin-induced angiogenesis. Biochemistry 28(18): 7401-7408.
- Kim JS, Soucek J, Matousek J, Raines RT (1995) Catalytic activity of bovine seminal ribonuclease is essential for its immunosuppressive and other biological activities. The Biochemical journal 308( Pt 2): 547-550.
- Strydom DJ (1998) The angiogenins. Cellular and molecular life sciences 54(8): 811-824.
- Leland PA, Staniszewski KE, Park C, Kelemen BR, Raines RT (2002) The ribonucleolytic activity of angiogenin. Biochemistry 41(4): 1343-1350.
- Ulyanova V, Vershinina V, Ilinskaya O (2011) Barnase and binase: twins with distinct fates. The FEBS journal 278(19): 3633-3643.
- Makarov AA, Kolchinsky A, Ilinskaya ON (2008) Binase and other microbial RNases as potential anticancer agents. BioEssays : news and reviews in molecular, cellular and developmental biology 30(8): 781-790.
- Ardelt W, Ardelt B, Darzynkiewicz Z (2009) Ribonucleases as potential modalities in anticancer therapy. Eur J Pharmacol 625(1-3): 181-189.
- Smolewski P, Witkowska M, Zwolinska M, Cebula Obrzut B, Majchrzak A (2014) Cytotoxic activity of the amphibian ribonucleases onconase and r-amphinase on tumor cells from B cell lymphoproliferative disorders. Int J Oncol 45(1): 419-425.
- Wang XM, Guo ZY (2013) Recombinant expression, different downstream processing of the disulfide-rich anti-tumor peptide Ranpirnase and its effect on the growth of human glioma cell line SHG-44. Biomedical reports 1(5): 747-750.
- Fiorini C, Cordani M, Gotte G, Picone D, Donadelli M (2015) Onconase induces autophagy sensitizing pancreatic cancer cells to gemcitabine and activates Akt/mTOR pathway in a ROS-dependent manner. Biochim Biophys Acta 1853(3): 549-560.
- Raineri A, Prodomini S, Fasoli S, Gotte G, Menegazzi M (2019) Influence of onconase in the therapeutic potential of PARP inhibitors in A375 malignant melanoma cells. Biochem Pharmacol 167: 173-181.
- Raineri A, Fasoli S, Campagnari R, Gotte G, Menegazzi M (2019) Onconase Restores Cytotoxicity in Dabrafenib-Resistant A375 Human Melanoma Cells and Affects Cell Migration, Invasion and Colony Formation Capability. International journal of molecular sciences 20(23).
- Lee I, Lee YH, Mikulski SM, Shogen K (2003) Effect of ONCONASE +/- tamoxifen on ASPC1 human pancreatic tumors in nude mice. Adv Exp Med Biol 530: 187-196.
- Mikulski SM, Viera A, Ardelt W, Menduke H, Shogen K (1990) Tamoxifen and trifluoroperazine (Stelazine) potentiate cytostatic/cytotoxic effects of P-30 protein, a novel protein possessing anti-tumor activity. Cell Tissue Kinet 23(3): 237-246.
- Mikulski SM, Viera A, Darzynkiewicz Z, Shogen K (1992) Synergism between a novel amphibian oocyte ribonuclease and lovastatin in inducing cytostatic and cytotoxic effects in human lung and pancreatic carcinoma cell lines. British journal of cancer 66(2): 304-310.
- Mikulski SM, Costanzi JJ, Vogelzang NJ, McCachren S, Taub RN (2002) Phase II trial of a single weekly intravenous dose of ranpirnase in patients with unresectable malignant mesothelioma. J Clin Oncol 20(1): 274-281.
- Vogelzang NJ, Porta C, Mutti L (2005) New agents in the management of advanced mesothelioma. Semin Oncol 32(3): 336-350.
- Beck AK, Pass HI, Carbone M, Yang H (2008) Ranpirnase as a potential antitumor ribonuclease treatment for mesothelioma and other malignancies. Future Oncol 4(3): 341-349.
- Vasandani VM, Burris JA, Sung C (1999) Reversible nephrotoxicity of onconase and effect of lysine pH on renal onconase uptake. Cancer Chemother Pharmacol 44(2): 164-169.
- Leich F, Stöhr N, Rietz A, Ulbrich Hofmann R, Arnold U (2007) Endocytotic internalization as a crucial factor for the cytotoxicity of ribonucleases. The Journal of biological chemistry 282(38): 27640-27646.
- Wu Y, Mikulski SM, Ardelt W, Rybak SM, Youle RJ (1993) A cytotoxic ribonuclease. Study of the mechanism of onconase cytotoxicity. The Journal of biological chemistry 268(14): 10686-10693.
- Haigis MC, Raines RT (2003) Secretory ribonucleases are internalized by a dynaminindependent endocytic pathway. Journal of cell science 116(Pt 2): 313-324.
- Sundlass NK, Eller CH, Cui Q, Raines RT (2013) Contribution of electrostatics to the binding of pancreatic-type ribonucleases to membranes. Biochemistry 52(37): 6304-6312.
- Notomista E, Mancheño JM, Crescenzi O, Di Donato A, Gavilanes J (2006) The role of electrostatic interactions in the antitumor activity of dimeric RNases. The FEBS journal 273(16): 3687-3697.
- Turcotte RF, Lavis LD, Raines RT (2009) Onconase cytotoxicity relies on the distribution of its positive charge. The FEBS journal 276(14): 3846-3857.
- D Errico G, Ercole C, Lista M, Pizzo E, Falanga A, et al. (2011) Enforcing the positive charge of N-termini enhances membrane interaction and antitumor activity of bovine seminal ribonuclease. Biochimica et biophysica acta 1808(12): 3007-3015.
- Sundlass NK, Raines RT (2011) Arginine residues are more effective than lysine residues in eliciting the cellular uptake of onconase. Biochemistry 50(47): 10293-10299.
- Johnson RJ, Chao TY, Lavis LD, Raines RT (2007) Cytotoxic ribonucleases: the dichotomy of Coulombic forces. Biochemistry 46(36): 10308-10316.
- Rodríguez M, Torrent G, Bosch M, Rayne F, Dubremetz JF, et al. (2007) Intracellular pathway of Onconase that enables its delivery to the cytosol. Journal of cell science 120(Pt 8):1405-1411.
- Kobe B, Deisenhofer J (1993) Crystal structure of porcine ribonuclease inhibitor, a protein with leucine-rich repeats. Nature 366(6457): 751-756.
- Blackburn P (1979) Ribonuclease inhibitor from human placenta: rapid purification and assay. The Journal of biological chemistry 254(24): 12484-12487.
- Furia A, Moscato M, Calì G, Pizzo E, Confalone E, et al. (2011) The ribonuclease/angiogenin inhibitor is also present in mitochondria and nuclei. FEBS letters 585(4): 613-617.
- Kobe B, Deisenhofer J (1995) A structural basis of the interactions between leucine-rich repeats and protein ligands. Nature 374: 183-186.
- Johnson RJ, McCoy JG, Bingman CA, Phillips GN, Raines RT (2007) Inhibition of human pancreatic ribonuclease by the human ribonuclease inhibitor protein. Journal of molecular biology 368(2): 434-449.
- Papageorgiou AC, Shapiro R, Acharya KR (1997) Molecular recognition of human angiogenin by placental ribonuclease inhibitor--an X-ray crystallographic study at 2.0 A resolution. The EMBO journal 16(17): 5162-5177.
- Iyer S, Holloway DE, Kumar K, Shapiro R, Acharya KR (2005) Molecular recognition of human eosinophil-derived neurotoxin (RNase 2) by placental ribonuclease inhibitor. Journal of molecular biology 347(3): 637-655.
- Rutkoski TJ, Raines RT (2008) Evasion of ribonuclease inhibitor as a determinant of ribonuclease cytotoxicity. Curr Pharm Biotechnol 9(3): 185-189.
- Kobe B, Deisenhofer J (1996) Mechanism of ribonuclease inhibition by ribonuclease inhibitor protein based on the crystal structure of its complex with ribonuclease A. Journal of molecular biology 264: 1028-1043.
- Lomax JE, Bianchetti CM, Chang A, Phillips GN, Fox BG (2014) Functional evolution of ribonuclease inhibitor: insights from birds and reptiles. Journal of molecular biology 426(17): 3041-3056.
- Saxena SK, Sirdeshmukh R, Ardelt W, Mikulski SM, Shogen K (2002) Entry into cells and selective degradation of tRNAs by a cytotoxic member of the RNase A family. The Journal of biological chemistry 277(17): 15142-15146.
- Antignani A, Naddeo M, Cubellis MV, Russo A, D Alessio G (2001) Antitumor action of seminal ribonuclease, its dimeric structure, and its resistance to the cytosolic ribonuclease inhibitor. Biochemistry 40(12): 3492-3496.
- Gotte G, Laurents DV, Merlino A, Picone D, Spadaccini R (2013) Structural and functional relationships of natural and artificial dimeric bovine ribonucleases: New scaffolds for potential antitumor drugs. FEBS letters 587(22): 3601-3608.
- Murthy BS, Sirdeshmukh R (1992) Sensitivity of monomeric and dimeric forms of bovine seminal ribonuclease to human placental ribonuclease inhibitor. The Biochemical journal 281(Pt 2): 343-348.
- Kim JS, Soucek J, Matousek J, Raines RT (1995) Mechanism of ribonuclease cytotoxicity. The Journal of biological chemistry 270(52): 31097-31102.
- Monti DM, D Alessio G (2004) Cytosolic RNase inhibitor only affects RNases with intrinsic cytotoxicity. The Journal of biological chemistry 279(38): 39195-39198.
- Naddeo M, Vitagliano L, Russo A, Gotte G, D Alessio G (2005) Interactions of the cytotoxic RNase A dimers with the cytosolic ribonuclease inhibitor. FEBS letters 579(12): 2663-2668.
- Fiorini C, Gotte G, Donnarumma F, Picone D, Donadelli M (2014) Bovine seminal ribonuclease triggers Beclin1-mediated autophagic cell death in pancreatic cancer cells. Biochim Biophys Acta 1843(5): 976-984.
- Matousek J, Gotte G, Pouckova P, Soucek J, Slavik T, et al. (2003) Antitumor activity and other biological actions of oligomers of ribonuclease A. The Journal of biological chemistry 278(26): 23817-23822.
- Libonati M, Gotte G, Vottariello F (2008) A novel biological actions acquired by ribonuclease through oligomerization. Curr Pharm Biotechnol 9(3): 200-209.
- Marianayagam NJ, Sunde M, Matthews JM (2004) The power of two: protein dimerization in biology. Trends Biochem Sci 29(11): 618-625.
- Kumari N, Yadav S (2019) Modulation of protein oligomerization: An overview. Progress in biophysics and molecular biology 149: 99-113.
- Berisio R, Sica F, De Lorenzo C, Di Fiore A, Piccoli R, et al. (2003) Crystal structure of the dimeric unswapped form of bovine seminal ribonuclease. FEBS letters 554(1-2): 105-110.
- Wang D, Wilson G, Moore S (1976) Preparation of cross-linked dimers of pancreatic ribonuclease. Biochemistry 15(3): 660-665.
- Di Donato A, Cafaro V, D Alessio G (1994) Ribonuclease A can be transformed into a dimeric ribonuclease with antitumor activity. The Journal of biological chemistry 269(26): 17394-17396.
- Gotte G, Testolin L, Costanzo C, Sorrentino S, Armato U, et al. (1997) Cross-linked trimers of bovine ribonuclease A: activity on double-stranded RNA and antitumor action. FEBS letters 415(3): 308-312.
- Rutkoski TJ, Kink JA, Strong LE, Schilling CI, Raines RT (2010) Antitumor activity of ribonuclease multimers created by site-specific covalent tethering. Bioconjugate chemistry 21: 1691-1702.
- De Lorenzo C, D Alessio G (2008) From immunotoxins to immunoRNases. Curr Pharm Biotechnol 9(3): 210-214.
- De Lorenzo C, D Alessio G (2009) Human anti-ErbB2 immunoagents--immunoRNases and compact antibodies. The FEBS journal 276(6): 1527-1535.
- Gotte G, Menegazzi M (2019) Biological Activities of Secretory RNases: Focus on Their Oligomerization to Design Antitumor Drugs. Frontiers in immunology 10: 2626.
- Zhao HL, Xue C, Du JL, Ren M, Xia S, et al. (2012) Sustained and cancer cell targeted cytosolic delivery of Onconase results in potent antitumor effects. Journal of controlled release 159(3): 346-352.
- Kiesgen S, Liebers N, Cremer M, Arnold U, Weber T, et al. (2014) A fusogenic dengue virus-derived peptide enhances antitumor efficacy of an antibody-ribonuclease fusion protein targeting the EGF receptor. Protein Eng Des Sel 27(10): 331-337.
- Qi J, Ye X, Li L, Bai H, Xu C (2018) Improving the specific antitumor efficacy of ONC by fusion with N-terminal domain of transferrin. Biosci Biotechnol Biochem 82(7): 1153-1158.
- Kiesgen S, Arndt MAE, Körber C, Arnold U, Weber T, et al. (2015) An EGF receptor targeting Ranpirnase-diabody fusion protein mediates potent antitumour activity in vitro and in vivo. Cancer letters 357(1): 364-373.
- Mathew M, Verma RS (2009) Humanized immunotoxins: a new generation of immunotoxins for targeted cancer therapy. Cancer science 100(8): 1359-1365.
- Messmann RA, Vitetta ES, Headlee D, Senderowicz AM, Figg WD, et al. (2000) A phase I study of combination therapy with immunotoxins IgG-HD37-deglycosylated ricin A chain (dgA) and IgG-RFB4-dgA (Combotox) in patients with refractory CD19(+), CD22(+) B cell lymphoma. Clin Cancer Res 6(4): 1302-1313.
- Krauss J, Arndt MA, Vu BK, Newton DL, Seeber S (2005) Efficient killing of CD22+ tumor cells by a humanized diabody-RNase fusion protein. Biochem Biophys Res Commun 331(2): 595-602.
- Gronenborn AM (2009) Protein acrobatics in pairs--dimerization via domain swapping. Curr Opin Struct Biol 19(1): 39-49.
- Bennett MJ, Schlunegger MF, Eisenberg D (1995) 3D domain swapping: a mechanism for oligomer assembly. Protein science 4(12): 2455-2468.
- Yang S, Cho SS, Levy Y, Cheung MS, Levine H (2004) Domain swapping is a consequence of minimal frustration. Proc Natl Acad Sci U S A 101(38): 13786-13791.
- Rousseau F, Schymkowitz J, Itzhaki LS (2012) Implications of 3D domain swapping for protein folding, misfolding and function. Adv Exp Med Biol 747: 137-152.
- Mackinnon SS, Malevanets A, Wodak SJ (2013) Intertwined associations in structures of homooligomeric proteins. Structure 21(4): 638-649.
- Bennett MJ, Choe S, Eisenberg D (1994) Domain swapping: entangling alliances between proteins. Proc Natl Acad Sci U S A 91(8): 3127-3131.
- Liu Y, Eisenberg D (2002) 3D domain swapping: as domains continue to swap. Protein science 11(6): 1285-1299.
- Bennett MJ, Sawaya MR, Eisenberg D (2006) Deposition diseases and 3D domain swapping. Structure 14(5): 811-824.
- Anfinsen CB (1973) Principles that govern the folding of protein chains. Science 181(4096): 223-230.
- Libonati M, Gotte G (2004) Oligomerization of bovine ribonuclease A: structural and functional features of its multimers. The Biochemical journal 380(Pt 2): 311-327.
- Cozza G, Moro S, Gotte G (2008) Elucidation of the ribonuclease a aggregation process mediated by 3D domain swapping: a computational approach reveals possible new multimeric structures. Biopolymers 89(1): 26-39.
- Gotte G, Mahmoud Helmy A, Ercole C, Spadaccini R, Laurents DV, et al. (2012) Double domain swapping in bovine seminal RNase: formation of distinct N- and C-swapped tetramers and multimers with increasing biological activities. PloS one 7(10): e46804.
- Titani K, Takio K, Kuwada M, Nitta K, Sakakibara F, et al. (1987) Amino acid sequence of sialic acid binding lectin from frog (Rana catesbeiana) eggs. Biochemistry 26(8): 2189-2194.
- Suhasini AN, Sirdeshmukh R (2007) Onconase action on tRNA(Lys3), the primer for HIV-1 reverse transcription. Biochem Biophys Res Commun 363(2): 304-309.
- Qiao M, Zu LD, He XH, Shen RL, Wang QC, et al. (2012) Onconase downregulates microRNA expression through targeting microRNA precursors. Cell research 22(7): 1199-1202.
- Fagagnini A, Pica A, Fasoli S, Montioli R, Donadelli M, et al. (2017) Onconase dimerization through 3D domain swapping: structural investigations and increase in the apoptotic effect in cancer cells. The Biochemical journal 474(22): 3767-3781.
- Crestfield AM, Stein WH, Moore S (1962) On the aggregation of bovine pancreatic ribonuclease. Archives of biochemistry and biophysics Suppl 1: 217-222.
- Liu Y, Hart PJ, Schlunegger MP, Eisenberg D (1998) The crystal structure of a 3D domainswapped dimer of RNase A at a 2.1-A resolution. Proc Natl Acad Sci U S A 95(7): 3437-3442.
- Leland PA, Staniszewski KE, Kim B, Raines RT (2000) A synapomorphic disulfide bond is critical for the conformational stability and cytotoxicity of an amphibian ribonuclease. FEBS letters 477(3): 203-207.
- Notomista E, Catanzano F, Graziano G, Di Gaetano S, Barone G, et al. (2001) Contribution of chain termini to the conformational stability and biological activity of onconase. Biochemistry 40(31): 9097-9103.
- Benito A, Laurents DV, Ribó M, Vilanova M (2008) The structural determinants that lead to the formation of particular oligomeric structures in the pancreatic-type ribonuclease family. Curr Protein Pept Sci 9(4): 370-393.
- Gotte G, Vottariello F, Libonati M (2003) Thermal aggregation of ribonuclease A. A contribution to the understanding of the role of 3D domain swapping in protein aggregation. J Biol Chem 278(12): 10763-10769.
- Gotte G, Donadelli M, Laurents DV, Vottariello F, Morbio M, et al. (2006) Increase of RNase a N-terminus polarity or C-terminus apolarity changes the two domains' propensity to swap and form the two dimeric conformers of the protein. Biochemistry 45(36): 10795-10806.
- Esposito L, Donnarumma F, Ruggiero A, Leone S, Vitagliano L, et al. (2019) Structure, stability and aggregation propensity of a Ribonuclease A-Onconase chimera. International journal of biological macromolecules 133: 1125-1133.
- Liu Y, Gotte G, Libonati M, Eisenberg D (2001) A domain-swapped RNase A dimer with implications for amyloid formation. Nat Struct Biol 8(3): 211-214.
- Lopez Alonso JP, Diez Garcia F, Font J, Ribo M, Vilanova, M, et al. (2009) Carbodiimide EDC induces cross-links that stabilize RNase A C-dimer against dissociation: EDC adducts can affect protein net charge, conformation, and activity. Bioconjugate chemistry 20(8): 1459-1473.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.